Virion endocytosis is a major target for murid herpesvirus-4 neutralization
- PMID: 22377583
- PMCID: PMC3755512
- DOI: 10.1099/vir.0.040790-0
Virion endocytosis is a major target for murid herpesvirus-4 neutralization
Abstract
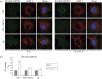
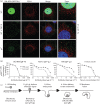
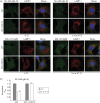
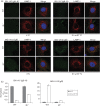
Herpesviruses consistently transmit from immunocompetent carriers, implying that their neutralization is hard to achieve. Murid herpesvirus-4 (MuHV-4) exploits host IgG Fc receptors to bypass blocks to cell binding, and pH-dependent protein conformation changes to unveil its fusion machinery only after endocytosis. Nevertheless, neutralization remains possible by targeting the virion glycoprotein H (gH)-gL heterodimer, and the neutralizing antibody responses of MuHV-4 carriers are improved by boosting with recombinant gH-gL. We analysed here how gH-gL-directed neutralization works. The MuHV-4 gH-gL binds to heparan sulfate. However, most gH-gL-specific neutralizing antibodies did not block this interaction; neither did they act directly on fusion. Instead, they blocked virion endocytosis and transport to the late endosomes, where membrane fusion normally occurs. The poor endocytosis of gH-gL-neutralized virions was recapitulated precisely by virions genetically lacking gL. Therefore, driving virion uptake appears to be an important function of gH-gL that provides a major target for antibody-mediated neutralization.
Figures



Similar articles
-
Bovine herpesvirus type 4 glycoprotein L is nonessential for infectivity but triggers virion endocytosis during entry.J Virol. 2012 Mar;86(5):2653-64. doi: 10.1128/JVI.06238-11. Epub 2011 Dec 28. J Virol. 2012. PMID: 22205754 Free PMC article.
-
Glycoprotein L sets the neutralization profile of murid herpesvirus 4.J Gen Virol. 2009 May;90(Pt 5):1202-1214. doi: 10.1099/vir.0.008755-0. Epub 2009 Mar 4. J Gen Virol. 2009. PMID: 19264603 Free PMC article.
-
Murine gammaherpesvirus-68 glycoprotein H-glycoprotein L complex is a major target for neutralizing monoclonal antibodies.J Gen Virol. 2006 Jun;87(Pt 6):1465-1475. doi: 10.1099/vir.0.81760-0. J Gen Virol. 2006. PMID: 16690911
-
Immune control of mammalian gamma-herpesviruses: lessons from murid herpesvirus-4.J Gen Virol. 2009 Oct;90(Pt 10):2317-2330. doi: 10.1099/vir.0.013300-0. Epub 2009 Jul 15. J Gen Virol. 2009. PMID: 19605591 Review.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
Cited by
-
Deletion of Murid Herpesvirus 4 ORF63 Affects the Trafficking of Incoming Capsids toward the Nucleus.J Virol. 2015 Dec 16;90(5):2455-72. doi: 10.1128/JVI.02942-15. J Virol. 2015. PMID: 26676769 Free PMC article.
-
Glycoprotein B cleavage is important for murid herpesvirus 4 to infect myeloid cells.J Virol. 2013 Oct;87(19):10828-42. doi: 10.1128/JVI.00709-13. Epub 2013 Jul 31. J Virol. 2013. PMID: 23903840 Free PMC article.
-
A heparan-dependent herpesvirus targets the olfactory neuroepithelium for host entry.PLoS Pathog. 2012;8(11):e1002986. doi: 10.1371/journal.ppat.1002986. Epub 2012 Nov 1. PLoS Pathog. 2012. PMID: 23133384 Free PMC article.
-
Antibody-mediated control mechanisms of viral infections.Immunol Rev. 2024 Nov;328(1):205-220. doi: 10.1111/imr.13383. Epub 2024 Aug 20. Immunol Rev. 2024. PMID: 39162394 Review.
-
A new insight into viral proteins as Immunomodulatory therapeutic agents: KSHV vOX2 a homolog of human CD200 as a potent anti-inflammatory protein.Iran J Basic Med Sci. 2016 Jan;19(1):2-13. Iran J Basic Med Sci. 2016. PMID: 27096058 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

