c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains
- PMID: 22378042
- PMCID: PMC3314471
- DOI: 10.1172/JCI61143
c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains
Abstract
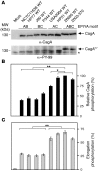
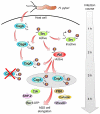
Many bacterial pathogens inject into host cells effector proteins that are substrates for host tyrosine kinases such as Src and Abl family kinases. Phosphorylated effectors eventually subvert host cell signaling, aiding disease development. In the case of the gastric pathogen Helicobacter pylori, which is a major risk factor for the development of gastric cancer, the only known effector protein injected into host cells is the oncoprotein CagA. Here, we followed the hierarchic tyrosine phosphorylation of H. pylori CagA as a model system to study early effector phosphorylation processes. Translocated CagA is phosphorylated on Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs EPIYA-A, EPIYA-B, and EPIYA-C in Western strains of H. pylori and EPIYA-A, EPIYA-B, and EPIYA-D in East Asian strains. We found that c-Src only phosphorylated EPIYA-C and EPIYA-D, whereas c-Abl phosphorylated EPIYA-A, EPIYA-B, EPIYA-C, and EPIYA-D. Further analysis revealed that CagA molecules were phosphorylated on 1 or 2 EPIYA motifs, but never simultaneously on 3 motifs. Furthermore, none of the phosphorylated EPIYA motifs alone was sufficient for inducing AGS cell scattering and elongation. The preferred combination of phosphorylated EPIYA motifs in Western strains was EPIYA-A and EPIYA-C, either across 2 CagA molecules or simultaneously on 1. Our study thus identifies a tightly regulated hierarchic phosphorylation model for CagA starting at EPIYA-C/D, followed by phosphorylation of EPIYA-A or EPIYA-B. These results provide insight for clinical H. pylori typing and clarify the role of phosphorylated bacterial effector proteins in pathogenesis.
Figures






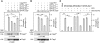
Comment in
-
Multistep activation of the Helicobacter pylori effector CagA.J Clin Invest. 2012 Apr;122(4):1192-5. doi: 10.1172/JCI61578. Epub 2012 Mar 1. J Clin Invest. 2012. PMID: 22378039 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

