Penetrance of biallelic SMARCAL1 mutations is associated with environmental and genetic disturbances of gene expression
- PMID: 22378147
- PMCID: PMC3349428
- DOI: 10.1093/hmg/dds083
Penetrance of biallelic SMARCAL1 mutations is associated with environmental and genetic disturbances of gene expression
Abstract
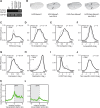
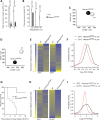
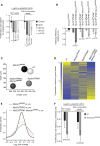
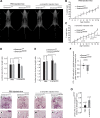
Biallelic mutations of the DNA annealing helicase SMARCAL1 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a-like 1) cause Schimke immuno-osseous dysplasia (SIOD, MIM 242900), an incompletely penetrant autosomal recessive disorder. Using human, Drosophila and mouse models, we show that the proteins encoded by SMARCAL1 orthologs localize to transcriptionally active chromatin and modulate gene expression. We also show that, as found in SIOD patients, deficiency of the SMARCAL1 orthologs alone is insufficient to cause disease in fruit flies and mice, although such deficiency causes modest diffuse alterations in gene expression. Rather, disease manifests when SMARCAL1 deficiency interacts with genetic and environmental factors that further alter gene expression. We conclude that the SMARCAL1 annealing helicase buffers fluctuations in gene expression and that alterations in gene expression contribute to the penetrance of SIOD.
Figures



References
-
- Romaschoff D.D. Über die Variabilität in der Manifestierung eines erblichen Merkmales (Abdomen abnormalis) bei Drosophila funebris F. J. Psychol. Neurol. 1925;31:323–325.
-
- Timoféeff-Ressovsky N.W. Über den Einfluss des Genotypus auf das phänotypen Auftreten eines einzelnes Gens. J. Psychol. Neurol. 1925;31:305–310.
-
- Vogt O. Psychiatrisch wichtige Tatsachen der zoologisch-botanischen Systematik. Zeitschrift für die gesamte. Neurol. Psychiatr. (Bucur) 1926;101:805–832.
-
- Zlotogora J. Penetrance and expressivity in the molecular age. Genet. Med. 2003;5:347–352. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

