Diverse levels of an inwardly rectifying potassium conductance generate heterogeneous neuronal behavior in a population of dorsal cochlear nucleus pyramidal neurons
- PMID: 22378165
- PMCID: PMC3378370
- DOI: 10.1152/jn.00660.2011
Diverse levels of an inwardly rectifying potassium conductance generate heterogeneous neuronal behavior in a population of dorsal cochlear nucleus pyramidal neurons
Abstract
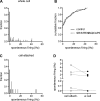
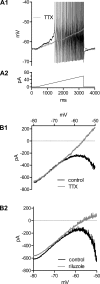
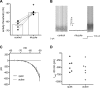
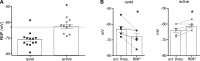
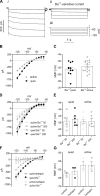
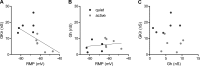
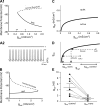
Homeostatic mechanisms maintain homogeneous neuronal behavior among neurons that exhibit substantial variability in the expression levels of their ionic conductances. In contrast, the mechanisms, which generate heterogeneous neuronal behavior across a neuronal population, remain poorly understood. We addressed this problem in the dorsal cochlear nucleus, where principal neurons exist in two qualitatively distinct states: spontaneously active or not spontaneously active. Our studies reveal that distinct activity states are generated by the differential levels of a Ba(2+)-sensitive, inwardly rectifying potassium conductance (K(ir)). Variability in K(ir) maximal conductance causes variations in the resting membrane potential (RMP). Low K(ir) conductance depolarizes RMP to voltages above the threshold for activating subthreshold-persistent sodium channels (Na(p)). Once Na(p) channels are activated, the RMP becomes unstable, and spontaneous firing is triggered. Our results provide a biophysical mechanism for generating neural heterogeneity, which may play a role in the encoding of sensory information.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

