Modulating locomotor adaptation with cerebellar stimulation
- PMID: 22378177
- PMCID: PMC3378372
- DOI: 10.1152/jn.00645.2011
Modulating locomotor adaptation with cerebellar stimulation
Abstract
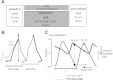
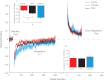
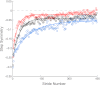
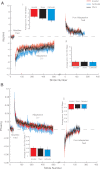
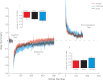
Human locomotor adaptation is necessary to maintain flexibility of walking. Several lines of research suggest that the cerebellum plays a critical role in motor adaptation. In this study we investigated the effects of noninvasive stimulation of the cerebellum to enhance locomotor adaptation. We found that anodal cerebellar transcranial direct current stimulation (tDCS) applied during adaptation expedited the adaptive process while cathodal cerebellar tDCS slowed it down, without affecting the rate of de-adaptation of the new locomotor pattern. Interestingly, cerebellar tDCS affected the adaptation rate of spatial but not temporal elements of walking. It may be that spatial and temporal control mechanisms are accessible through different neural circuits. Our results suggest that tDCS could be used as a tool to modulate locomotor training in neurological patients with gait impairments.
Figures
References
-
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, Fregni F. Effects of transcranial direct current stimulation on working memory in patients with Parkinson's disease. J Neurol Sci 249: 31–38, 2006 - PubMed
-
- Choi J, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci 10: 1055–1062, 2007 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

