Regulation of oxidative phosphorylation complex activity: effects of tissue-specific metabolic stress within an allometric series and acute changes in workload
- PMID: 22378775
- PMCID: PMC3362144
- DOI: 10.1152/ajpregu.00596.2011
Regulation of oxidative phosphorylation complex activity: effects of tissue-specific metabolic stress within an allometric series and acute changes in workload
Abstract
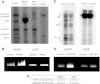
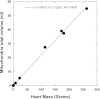
The concentration of mitochondrial oxidative phosphorylation complexes (MOPCs) is tuned to the maximum energy conversion requirements of a given tissue; however, whether the activity of MOPCs is altered in response to acute changes in energy conversion demand is unclear. We hypothesized that MOPCs activity is modulated by tissue metabolic stress to maintain the energy-metabolism homeostasis. Metabolic stress was defined as the observed energy conversion rate/maximum energy conversion rate. The maximum energy conversion rate was assumed to be proportional to the concentration of MOPCs, as determined with optical spectroscopy, gel electrophoresis, and mass spectrometry. The resting metabolic stress of the heart and liver across the range of resting metabolic rates within an allometric series (mouse, rabbit, and pig) was determined from MPOCs content and literature respiratory values. The metabolic stress of the liver was high and nearly constant across the allometric series due to the proportional increase in MOPCs content with resting metabolic rate. In contrast, the MOPCs content of the heart was essentially constant in the allometric series, resulting in an increasing metabolic stress with decreasing animal size. The MOPCs activity was determined in native gels, with an emphasis on Complex V. Extracted MOPCs enzyme activity was proportional to resting metabolic stress across tissues and species. Complex V activity was also shown to be acutely modulated by changes in metabolic stress in the heart, in vivo and in vitro. The modulation of extracted MOPCs activity suggests that persistent posttranslational modifications (PTMs) alter MOPCs activity both chronically and acutely, specifically in the heart. Protein phosphorylation of Complex V was correlated with activity inhibition under several conditions, suggesting that protein phosphorylation may contribute to activity modulation with energy metabolic stress. These data are consistent with the notion that metabolic stress modulates MOPCs activity in the heart.
Figures






Similar articles
-
Cholesterol enrichment in liver mitochondria impairs oxidative phosphorylation and disrupts the assembly of respiratory supercomplexes.Redox Biol. 2019 Jun;24:101214. doi: 10.1016/j.redox.2019.101214. Epub 2019 May 9. Redox Biol. 2019. PMID: 31108462 Free PMC article.
-
Programming and regulation of metabolic homeostasis.Am J Physiol Endocrinol Metab. 2015 Mar 15;308(6):E506-17. doi: 10.1152/ajpendo.00544.2014. Epub 2015 Jan 20. Am J Physiol Endocrinol Metab. 2015. PMID: 25605644
-
Bioenergetic functions in subpopulations of heart mitochondria are preserved in a non-obese type 2 diabetes rat model (Goto-Kakizaki).Sci Rep. 2020 Mar 25;10(1):5444. doi: 10.1038/s41598-020-62370-8. Sci Rep. 2020. PMID: 32214195 Free PMC article.
-
Mitochondrial Metabolism in Aging Heart.Circ Res. 2016 May 13;118(10):1593-611. doi: 10.1161/CIRCRESAHA.116.307505. Circ Res. 2016. PMID: 27174952 Free PMC article. Review.
-
Mitochondrial membrane transporters and metabolic switch in heart failure.Heart Fail Rev. 2019 Mar;24(2):255-267. doi: 10.1007/s10741-018-9756-2. Heart Fail Rev. 2019. PMID: 30535838 Review.
Cited by
-
Intracardiac light catheter for rapid scanning transmural absorbance spectroscopy of perfused myocardium: measurement of myoglobin oxygenation and mitochondria redox state.Am J Physiol Heart Circ Physiol. 2017 Dec 1;313(6):H1199-H1208. doi: 10.1152/ajpheart.00306.2017. Epub 2017 Sep 22. Am J Physiol Heart Circ Physiol. 2017. PMID: 28939647 Free PMC article.
-
MCU Overexpression Rescues Inotropy and Reverses Heart Failure by Reducing SR Ca2+ Leak.Circ Res. 2021 Apr 16;128(8):1191-1204. doi: 10.1161/CIRCRESAHA.120.318562. Epub 2021 Feb 1. Circ Res. 2021. PMID: 33522833 Free PMC article.
-
The Functional Impact of Mitochondrial Structure Across Subcellular Scales.Front Physiol. 2020 Nov 11;11:541040. doi: 10.3389/fphys.2020.541040. eCollection 2020. Front Physiol. 2020. PMID: 33262702 Free PMC article. Review.
-
Continuous monitoring of enzymatic activity within native electrophoresis gels: application to mitochondrial oxidative phosphorylation complexes.Anal Biochem. 2012 Dec 1;431(1):30-9. doi: 10.1016/j.ab.2012.08.023. Epub 2012 Sep 10. Anal Biochem. 2012. PMID: 22975200 Free PMC article.
-
Dysregulated Gene Expression in Lymphoblasts from Parkinson's Disease.Proteomes. 2022 Jun 1;10(2):20. doi: 10.3390/proteomes10020020. Proteomes. 2022. PMID: 35736800 Free PMC article.
References
-
- Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol 34: 1259–1271, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

