Measuring the firing rate of high-resistance neurons with cell-attached recording
- PMID: 22378885
- PMCID: PMC6622012
- DOI: 10.1523/JNEUROSCI.5371-11.2012
Measuring the firing rate of high-resistance neurons with cell-attached recording
Abstract
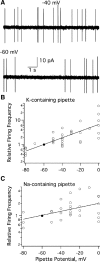
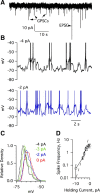
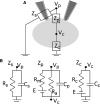
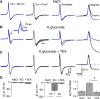
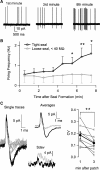
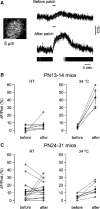
Cell-attached recording is extensively used to study the firing rate of mammalian neurons, but potential limitations of the method have not been investigated in detail. Here we perform cell-attached recording of molecular layer interneurons in cerebellar slices from rats and mice, and we study how experimental conditions influence the measured firing rate. We find that this rate depends on time in cell-attached mode, on pipette potential, and on pipette ionic composition. In the first minute after sealing, action currents are variable in shape and size, presumably reflecting membrane instability. The firing rate remains approximately constant during the first 4 min after sealing and gradually increases afterward. Making the pipette potential more positive leads to an increase in the firing rate, with a steeper dependence on voltage if the pipette solution contains K(+) as the main cation than if it contains Na(+). Ca(2+) imaging experiments show that establishing a cell-attached recording can result in an increased somatic Ca(2+) concentration, reflecting an increased firing rate linked to an increase in the pipette-cell conductance. Pipette effects on cell firing are traced to a combination of passive electrical coupling, opening of voltage- and Ca(2+)-sensitive K(+) channels (BK channels) after action potentials, and random activation of voltage-insensitive, presumably mechanosensitive, cationic channels. We conclude that, unless experimental conditions are optimized, cell-attached recordings in small neurons may report erroneous firing rates.
Figures

References
-
- Barbour B, Isope P. Combining loose cell-attached stimulation and recording. J Neurosci Methods. 2000;103:199–208. - PubMed
-
- Carter AG, Regehr WG. Quantal events shape cerebellar interneuron firing. Nat Neurosci. 2002;5:1309–1318. - PubMed
-
- Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol. 2009;10:44–52. - PubMed
-
- Chavas J, Forero ME, Collin T, Llano I, Marty A. Osmotic tension as a possible link between GABAA receptor activation and intracellular calcium elevation. Neuron. 2004;44:701–713. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
