Nicotinic receptor-mediated filtering of mitral cell responses to olfactory nerve inputs involves the α3β4 subtype
- PMID: 22378897
- PMCID: PMC3306821
- DOI: 10.1523/JNEUROSCI.5024-11.2012
Nicotinic receptor-mediated filtering of mitral cell responses to olfactory nerve inputs involves the α3β4 subtype
Abstract
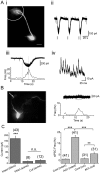
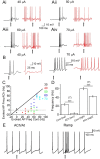
Acetylcholine (ACh) plays a major role in the processing of sensory inputs. Cholinergic input to the mammalian olfactory bulb modulates odor discrimination and perceptual learning by mechanisms that have yet to be elucidated. We have used the mouse olfactory bulb to examine the role of nicotinic ACh receptors (nAChRs) in regulating the responses of mitral cells (MCs), the output neurons of the olfactory bulb, to olfactory nerve input. We show that ACh activates α3β4* nAChRs (* denotes the possible presence of other subunits) on MCs, leading to their excitation. Despite depolarizing MCs directly, the net effect of nAChR activation is to suppress olfactory nerve-evoked responses in these cells via activity-dependent feedback GABAergic mechanisms. Our results indicate that nAChRs gate incoming olfactory nerve input wherein weak input stimuli are filtered out, whereas strong stimuli are transmitted via the MCs. Based on our observations, we provide a mechanistic model for the sharpening of MC receptive fields by nAChRs, which could aid in odor discrimination and perceptual learning.
Figures


Similar articles
-
A dominant role for the beta 4 nicotinic receptor subunit in nicotinic modulation of glomerular microcircuits in the mouse olfactory bulb.J Neurophysiol. 2018 Oct 1;120(4):2036-2048. doi: 10.1152/jn.00925.2017. Epub 2018 Aug 8. J Neurophysiol. 2018. PMID: 30089021 Free PMC article.
-
Nicotinic receptors modulate olfactory bulb external tufted cells via an excitation-dependent inhibitory mechanism.J Neurophysiol. 2013 Oct;110(7):1544-53. doi: 10.1152/jn.00865.2012. Epub 2013 Jul 10. J Neurophysiol. 2013. PMID: 23843430 Free PMC article.
-
Direct Recording of Dendrodendritic Excitation in the Olfactory Bulb: Divergent Properties of Local and External Glutamatergic Inputs Govern Synaptic Integration in Granule Cells.J Neurosci. 2017 Dec 6;37(49):11774-11788. doi: 10.1523/JNEUROSCI.2033-17.2017. Epub 2017 Oct 24. J Neurosci. 2017. PMID: 29066560 Free PMC article.
-
Acetylcholine and olfactory perceptual learning.Learn Mem. 2004 Jan-Feb;11(1):28-34. doi: 10.1101/lm.66404. Learn Mem. 2004. PMID: 14747514 Free PMC article. Review.
-
Functional organization of the main olfactory bulb.Microsc Res Tech. 1993 Feb 1;24(2):142-56. doi: 10.1002/jemt.1070240206. Microsc Res Tech. 1993. PMID: 8457726 Review.
Cited by
-
Top-down acetylcholine signaling via olfactory bulb vasopressin cells contributes to social discrimination in rats.Commun Biol. 2021 May 21;4(1):603. doi: 10.1038/s42003-021-02129-7. Commun Biol. 2021. PMID: 34021245 Free PMC article.
-
Perceptual spaces: mathematical structures to neural mechanisms.J Neurosci. 2013 Nov 6;33(45):17597-602. doi: 10.1523/JNEUROSCI.3343-13.2013. J Neurosci. 2013. PMID: 24198350 Free PMC article. Review.
-
Signaling between periglomerular cells reveals a bimodal role for GABA in modulating glomerular microcircuitry in the olfactory bulb.Proc Natl Acad Sci U S A. 2015 Jul 28;112(30):9478-83. doi: 10.1073/pnas.1424406112. Epub 2015 Jul 13. Proc Natl Acad Sci U S A. 2015. PMID: 26170298 Free PMC article.
-
Greater excitability and firing irregularity of tufted cells underlies distinct afferent-evoked activity of olfactory bulb mitral and tufted cells.J Physiol. 2014 May 15;592(10):2097-118. doi: 10.1113/jphysiol.2013.269886. Epub 2014 Mar 10. J Physiol. 2014. PMID: 24614745 Free PMC article.
-
Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb.J Neurosci. 2012 Jul 25;32(30):10105-16. doi: 10.1523/JNEUROSCI.0058-12.2012. J Neurosci. 2012. PMID: 22836246 Free PMC article.
References
-
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. - PubMed
-
- Ghatpande AS, Gelperin A. Presynaptic muscarinic receptors enhance glutamate release at the mitral/tufted to granule cell dendrodendritic synapse in the rat main olfactory bulb. J Neurophysiol. 2009;101:2052–2061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
