A protein array screen for Kaposi's sarcoma-associated herpesvirus LANA interactors links LANA to TIP60, PP2A activity, and telomere shortening
- PMID: 22379092
- PMCID: PMC3347335
- DOI: 10.1128/JVI.00169-12
A protein array screen for Kaposi's sarcoma-associated herpesvirus LANA interactors links LANA to TIP60, PP2A activity, and telomere shortening
Abstract
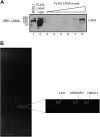
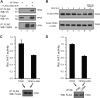
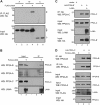
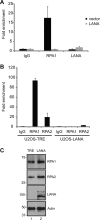
The Kaposi's sarcoma-associated herpesvirus (KSHV) LANA protein functions in latently infected cells as an essential participant in KSHV genome replication and as a driver of dysregulated cell growth. To identify novel LANA protein-cell protein interactions that could contribute to these activities, we performed a proteomic screen in which purified, adenovirus-expressed Flag-LANA protein was incubated with an array displaying 4,192 nonredundant human proteins. Sixty-one interacting cell proteins were consistently detected. LANA interactions with high-mobility group AT-hook 1 (HMGA1), HMGB1, telomeric repeat binding factor 1 (TRF1), xeroderma pigmentosum complementation group A (XPA), pygopus homolog 2 (PYGO2), protein phosphatase 2A (PP2A)B subunit, Tat-interactive protein 60 (TIP60), replication protein A1 (RPA1), and RPA2 proteins were confirmed in coimmunoprecipitation assays. LANA-associated TIP60 retained acetyltransferase activity and, unlike human papillomavirus E6 and HIV-1 TAT proteins, LANA did not reduce TIP60 stability. The LANA-bound PP2A B subunit was associated with the PP2A A subunit but not the catalytic C subunit, suggesting a disruption of PP2A phosphatase activity. This is reminiscent of the role of simian virus 40 (SV40) small t antigen. Chromatin immunoprecipitation (ChIP) assays showed binding of RPA1 and RPA2 to the KSHV terminal repeats. Interestingly, LANA expression ablated RPA1 and RPA2 binding to the cell telomeric repeats. In U2OS cells that rely on the alternative mechanism for telomere maintenance, LANA expression had minimal effect on telomere length. However, LANA expression in telomerase immortalized endothelial cells resulted in telomere shortening. In KSHV-infected cells, telomere shortening may be one more mechanism by which LANA contributes to the development of malignancy.
Figures
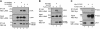


References
-
- An J, Sun Y, Rettig MB. 2004. Transcriptional coactivation of c-Jun by the KSHV-encoded LANA. Blood 103:222–228 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

