Signature biochemical properties of broadly cross-reactive HIV-1 neutralizing antibodies in human plasma
- PMID: 22379105
- PMCID: PMC3347347
- DOI: 10.1128/JVI.06547-11
Signature biochemical properties of broadly cross-reactive HIV-1 neutralizing antibodies in human plasma
Abstract
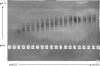
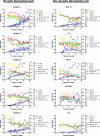
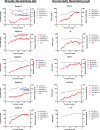
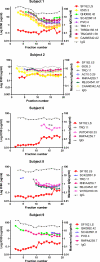
The common properties of broadly cross-reactive HIV-1 neutralization antibodies found in certain HIV-1-infected individuals holds significant value for understanding natural and vaccine-mediated anti-HIV immunity. Recent efforts have addressed this question by deriving neutralizing monoclonal anti-envelope antibodies from memory B cell pools of selected subjects. However, it has been more difficult to identify whether broadly neutralizing antibodies circulating in plasma possess shared characteristics among individuals. To address this question, we used affinity chromatography and isoelectric focusing to fractionate plasma immunoglobulin from 10 HIV-1-infected subjects (5 subjects with broad HIV-1 neutralizing activity and 5 controls). We find that plasma neutralizing activity typically partitions into at least two subsets of antibodies. Antibodies with restricted neutralization breadth have relatively neutral isoelectric points and preferentially bind to envelope monomers and trimers versus core antigens from which variable loops and other domains have been deleted. In comparison, broadly neutralizing antibodies account for a minor fraction of the total anti-envelope response. They are consistently distinguished by more basic isoelectric points and specificity for epitopes shared by monomeric gp120, gp120 core, or CD4-induced structures. Such biochemical properties might be exploited to reliably predict or produce broad anti-HIV immunity.
Figures

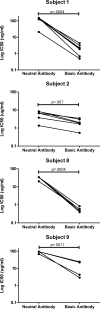
Similar articles
-
Broadly Neutralizing Antibodies against HIV: Back to Blood.Trends Mol Med. 2019 Mar;25(3):228-240. doi: 10.1016/j.molmed.2019.01.007. Epub 2019 Feb 18. Trends Mol Med. 2019. PMID: 30792120 Free PMC article. Review.
-
Evolution of cross-neutralizing antibody specificities to the CD4-BS and the carbohydrate cloak of the HIV Env in an HIV-1-infected subject.PLoS One. 2012;7(11):e49610. doi: 10.1371/journal.pone.0049610. Epub 2012 Nov 13. PLoS One. 2012. PMID: 23152926 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
-
Structural Features of Broadly Neutralizing Antibodies and Rational Design of Vaccine.Adv Exp Med Biol. 2018;1075:73-95. doi: 10.1007/978-981-13-0484-2_4. Adv Exp Med Biol. 2018. PMID: 30030790 Review.
Cited by
-
Near-Pan-neutralizing, Plasma Deconvoluted Antibody N49P6 Mimics Host Receptor CD4 in Its Quaternary Interactions with the HIV-1 Envelope Trimer.mBio. 2021 Aug 31;12(4):e0127421. doi: 10.1128/mBio.01274-21. Epub 2021 Jul 20. mBio. 2021. PMID: 34281393 Free PMC article.
-
λ Light Chain Bias Associated With Enhanced Binding and Function of Anti-HIV Env Glycoprotein Antibodies.J Infect Dis. 2016 Jan 1;213(1):156-64. doi: 10.1093/infdis/jiv448. Epub 2015 Sep 7. J Infect Dis. 2016. PMID: 26347575 Free PMC article.
-
Broadly Neutralizing Antibodies against HIV: Back to Blood.Trends Mol Med. 2019 Mar;25(3):228-240. doi: 10.1016/j.molmed.2019.01.007. Epub 2019 Feb 18. Trends Mol Med. 2019. PMID: 30792120 Free PMC article. Review.
-
Allosteric induction of the CD4-bound conformation of HIV-1 Gp120.Retrovirology. 2013 Dec 5;10:147. doi: 10.1186/1742-4690-10-147. Retrovirology. 2013. PMID: 24304511 Free PMC article.
-
Antibody cooperative adsorption onto AuNPs and its exploitation to force natural killer cells to kill HIV-infected T cells.Nano Today. 2021 Feb;36:101056. doi: 10.1016/j.nantod.2020.101056. Epub 2020 Dec 20. Nano Today. 2021. PMID: 34394703 Free PMC article.
References
-
- Amadori A, et al. 1990. IgG oligoclonal bands in sera of HIV-1 infected patients are mainly directed against HIV-1 determinants. AIDS Res. Hum. Retroviruses 6:581–586 - PubMed
-
- Biselli R, et al. 1996. Anti-V3 loop spectrotype in HIV-infected individuals during zidovudine therapy. Infection 24:227–233 - PubMed
-
- D'Amelio R, et al. 1992. Spectrotype of anti-gp120 antibodies remains stable during the course of HIV disease. J. Acquir. Immune Defic. Syndr. 5:930–935 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

