Pharmacological chaperoning of nicotinic acetylcholine receptors reduces the endoplasmic reticulum stress response
- PMID: 22379121
- PMCID: PMC3362896
- DOI: 10.1124/mol.112.077792
Pharmacological chaperoning of nicotinic acetylcholine receptors reduces the endoplasmic reticulum stress response
Abstract
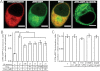
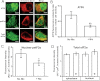
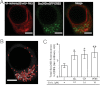
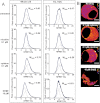
We report the first observation that endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) can decrease when a central nervous system drug acts as an intracellular pharmacological chaperone for its classic receptor. Transient expression of α4β2 nicotinic receptors (nAChRs) in Neuro-2a cells induced the nuclear translocation of activating transcription factor 6 (ATF6), which is part of the UPR. Cells were exposed for 48 h to the full agonist nicotine, the partial agonist cytisine, or the competitive antagonist dihydro-β-erythroidine; we also tested mutant nAChRs that readily exit the ER. Each of these four manipulations increased Sec24D-enhanced green fluorescent protein fluorescence of condensed ER exit sites and attenuated translocation of ATF6-enhanced green fluorescent protein to the nucleus. However, we found no correlation among the manipulations regarding other tested parameters [i.e., changes in nAChR stoichiometry (α4(2)β2(3) versus α4(3)β2(2)), changes in ER and trans-Golgi structures, or the degree of nAChR up-regulation at the plasma membrane]. The four manipulations activated 0 to 0.4% of nAChRs, which shows that activation of the nAChR channel did not underlie the reduced ER stress. Nicotine also attenuated endogenously expressed ATF6 translocation and phosphorylation of eukaryotic initiation factor 2α in mouse cortical neurons transfected with α4β2 nAChRs. We conclude that, when nicotine accelerates ER export of α4β2 nAChRs, this suppresses ER stress and the UPR. Suppression of a sustained UPR may explain the apparent neuroprotective effect that causes the inverse correlation between a person's history of tobacco use and susceptibility to developing Parkinson's disease. This suggests a novel mechanism for neuroprotection by nicotine.
Figures

References
-
- Ahmadi FA, Grammatopoulos TN, Poczobutt AM, Jones SM, Snell LD, Das M, Zawada WM. (2008) Dopamine selectively sensitizes dopaminergic neurons to rotenone-induced apoptosis. Neurochem Res 33:886–901 - PubMed
-
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. (2002) Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol 444:260–274 - PubMed
-
- Buisson B, Bertrand D. (2002) Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci 23:130–136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

