Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators
- PMID: 22379191
- PMCID: PMC3315118
- DOI: 10.1101/gad.183251.111
Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators
Abstract
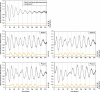
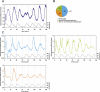
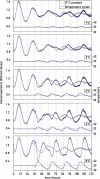
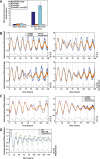
The circadian pacemaker in the suprachiasmatic nuclei (SCN) of the hypothalamus maintains phase coherence in peripheral cells through metabolic, neuronal, and humoral signaling pathways. Here, we investigated the role of daily body temperature fluctuations as possible systemic cues in the resetting of peripheral oscillators. Using precise temperature devices in conjunction with real-time monitoring of the bioluminescence produced by circadian luciferase reporter genes, we showed that simulated body temperature cycles of mice and even humans, with daily temperature differences of only 3°C and 1°C, respectively, could gradually synchronize circadian gene expression in cultured fibroblasts. The time required for establishing the new steady-state phase depended on the reporter gene, but after a few days, the expression of each gene oscillated with a precise phase relative to that of the temperature cycles. Smooth temperature oscillations with a very small amplitude could synchronize fibroblast clocks over a wide temperature range, and such temperature rhythms were also capable of entraining gene expression cycles to periods significantly longer or shorter than 24 h. As revealed by genetic loss-of-function experiments, heat-shock factor 1 (HSF1), but not HSF2, was required for the efficient synchronization of fibroblast oscillators to simulated body temperature cycles.
Figures



Similar articles
-
Rhythms of mammalian body temperature can sustain peripheral circadian clocks.Curr Biol. 2002 Sep 17;12(18):1574-83. doi: 10.1016/s0960-9822(02)01145-4. Curr Biol. 2002. PMID: 12372249
-
The systemic control of circadian gene expression.Diabetes Obes Metab. 2015 Sep;17 Suppl 1:23-32. doi: 10.1111/dom.12512. Diabetes Obes Metab. 2015. PMID: 26332965 Review.
-
Clock-Talk: Interactions between Central and Peripheral Circadian Oscillators in Mammals.Cold Spring Harb Symp Quant Biol. 2015;80:223-32. doi: 10.1101/sqb.2015.80.027490. Epub 2015 Dec 18. Cold Spring Harb Symp Quant Biol. 2015. PMID: 26683231 Review.
-
Steven A. Brown and the synchronization of circadian rhythms by body temperature cycles.Eur J Neurosci. 2024 Jul;60(2):3891-3900. doi: 10.1111/ejn.16431. Epub 2024 Jun 4. Eur J Neurosci. 2024. PMID: 38837456 Review.
-
Synchronization of circadian Per2 rhythms and HSF1-BMAL1:CLOCK interaction in mouse fibroblasts after short-term heat shock pulse.PLoS One. 2011;6(9):e24521. doi: 10.1371/journal.pone.0024521. Epub 2011 Sep 7. PLoS One. 2011. PMID: 21915348 Free PMC article.
Cited by
-
'Cold cuts' added to the circadian smorgasbord of regulatory mechanisms.Genes Dev. 2016 Sep 1;30(17):1909-10. doi: 10.1101/gad.289587.116. Genes Dev. 2016. PMID: 27664233 Free PMC article.
-
Using Circadian Rhythm Patterns of Continuous Core Body Temperature to Improve Fertility and Pregnancy Planning.J Circadian Rhythms. 2020 Sep 24;18:5. doi: 10.5334/jcr.200. J Circadian Rhythms. 2020. PMID: 33024445 Free PMC article. Review.
-
Circadian blueprint of metabolic pathways in the brain.Nat Rev Neurosci. 2019 Feb;20(2):71-82. doi: 10.1038/s41583-018-0096-y. Nat Rev Neurosci. 2019. PMID: 30559395 Free PMC article. Review.
-
Response to Stimulations Inducing Circadian Rhythm in Human Induced Pluripotent Stem Cells.Cells. 2020 Mar 4;9(3):620. doi: 10.3390/cells9030620. Cells. 2020. PMID: 32143467 Free PMC article.
-
Making Time: Conservation of Biological Clocks from Fungi to Animals.Microbiol Spectr. 2017 May;5(3):10.1128/microbiolspec.funk-0039-2016. doi: 10.1128/microbiolspec.FUNK-0039-2016. Microbiol Spectr. 2017. PMID: 28527179 Free PMC article. Review.
References
-
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP 2002. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol 12: 540–550 - PubMed
-
- Albrecht U, Sun ZS, Eichele G, Lee CC 1997. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91: 1055–1064 - PubMed
-
- Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U 2010. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142: 943–953 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
