Olanzapine causes a leptin-dependent increase in acetylcholine release in mouse prefrontal cortex
- PMID: 22379237
- PMCID: PMC3274332
- DOI: 10.5665/sleep.1686
Olanzapine causes a leptin-dependent increase in acetylcholine release in mouse prefrontal cortex
Abstract
Study objectives: The atypical antipsychotic olanzapine is used effectively for treating symptoms of schizophrenia and bipolar disorder. Unwanted effects of olanzapine include slowing of the electroencephalogram (EEG) during wakefulness and increased circulating levels of leptin. The mechanisms underlying the desired and undesired effects of olanzapine are poorly understood. Sleep and wakefulness are modulated by acetylcholine (ACh) in the prefrontal cortex, and leptin alters cholinergic transmission. This study tested the hypothesis that olanzapine interacts with leptin to regulate ACh release in the prefrontal cortex.
Design: Within/between subjects.
Setting: University of Michigan.
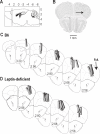
Patients or participants: Adult male C57BL/6J (B6) mice (n = 33) and B6.V-Lep(ob) (leptin-deficient) mice (n = 31).
Interventions: Olanzapine was delivered to the prefrontal cortex by microdialysis. Leptin-replacement in leptin-deficient mice was achieved using subcutaneous micro-osmotic pumps.
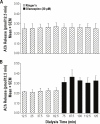
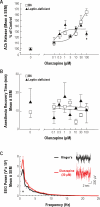
Measurements and results: Olanzapine caused a concentration-dependent increase in ACh release in B6 and leptin-deficient mice. Olanzapine was 230-fold more potent in leptin-deficient than in B6 mice for increasing ACh release, yet olanzapine caused a 51% greater ACh increase in B6 than in leptin-deficient mice. Olanzapine had no effect on recovery time from general anesthesia. Olanzapine increased EEG power in the delta (0.5-4 Hz) range. Thus, olanzapine dissociated the normal coupling between increased cortical ACh release, increased behavioral arousal, and EEG activation. Leptin replacement significantly enhanced (75%) the olanzapine-induced increase in ACh release.
Conclusion: Replacing leptin by systemic administration restored the olanzapine-induced enhancement of ACh release in the prefrontal cortex of leptin-deficient mouse.
Keywords: Atypical antipsychotics; B6.V-Lepob mouse; C57BL/6J mouse.
Figures

Similar articles
-
Prefrontal cortex acetylcholine release, EEG slow waves, and spindles are modulated by M2 autoreceptors in C57BL/6J mouse.J Neurophysiol. 2002 Jun;87(6):2817-22. doi: 10.1152/jn.2002.87.6.2817. J Neurophysiol. 2002. PMID: 12037184
-
Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal.J Neurosci. 2009 Jan 21;29(3):871-81. doi: 10.1523/JNEUROSCI.4111-08.2009. J Neurosci. 2009. PMID: 19158311 Free PMC article.
-
M2 muscarinic autoreceptors modulate acetylcholine release in prefrontal cortex of C57BL/6J mouse.J Pharmacol Exp Ther. 2001 Dec;299(3):960-6. J Pharmacol Exp Ther. 2001. PMID: 11714883
-
Postsynaptic muscarinic M1 receptors activate prefrontal cortical EEG of C57BL/6J mouse.J Neurophysiol. 2002 Dec;88(6):3003-9. doi: 10.1152/jn.00318.2002. J Neurophysiol. 2002. PMID: 12466425
-
Unraveling monoamine receptors involved in the action of typical and atypical antipsychotics on glutamatergic and serotonergic transmission in prefrontal cortex.Curr Pharm Des. 2010;16(5):502-15. doi: 10.2174/138161210790361416. Curr Pharm Des. 2010. PMID: 19909228 Review.
Cited by
-
Neurotransmitter networks in mouse prefrontal cortex are reconfigured by isoflurane anesthesia.J Neurophysiol. 2020 Jun 1;123(6):2285-2296. doi: 10.1152/jn.00092.2020. Epub 2020 Apr 29. J Neurophysiol. 2020. PMID: 32347157 Free PMC article.
-
Olanzapine Induces Inflammation and Immune Response via Activating ER Stress in the Rat Prefrontal Cortex.Curr Med Sci. 2021 Aug;41(4):788-802. doi: 10.1007/s11596-021-2401-7. Epub 2021 Aug 17. Curr Med Sci. 2021. PMID: 34403105
-
Chronic treatment with olanzapine increases adiposity by changing fuel substrate and causes desensitization of the acute metabolic side effects.Naunyn Schmiedebergs Arch Pharmacol. 2014 Feb;387(2):185-95. doi: 10.1007/s00210-013-0933-5. Epub 2013 Nov 5. Naunyn Schmiedebergs Arch Pharmacol. 2014. PMID: 24190543
-
Neuropharmacology of Sleep and Wakefulness: 2012 Update.Sleep Med Clin. 2012 Sep 1;7(3):469-486. doi: 10.1016/j.jsmc.2012.06.010. Epub 2012 Sep 4. Sleep Med Clin. 2012. PMID: 23162386 Free PMC article.
References
-
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. - PubMed
-
- Cipriani A, Rendell J, Geddes JR. Olanzapine in the long-term treatment of bipolar disorder: a systematic review and meta-analysis. J Psychopharmacol. 2010;24:1729–38. - PubMed
-
- Keck PE., Jr Bipolar depression: a new role for atypical antipsychotics? Bipolar Disord. 2005;7(Suppl 4):34–40. - PubMed
-
- Roth BL, Sheffler D, Potkin SG. Atypical antipsychotic drug actions: unitary or multiple mechanisms for ‘atypicality’? Clin Neurosci Res. 2003;3:108–17.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

