Cthrc1 is a negative regulator of myelination in Schwann cells
- PMID: 22379615
- PMCID: PMC3505855
- DOI: 10.1002/glia.22273
Cthrc1 is a negative regulator of myelination in Schwann cells
Abstract
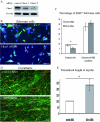
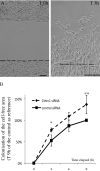
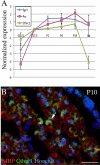
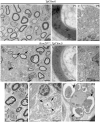
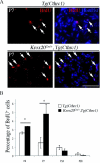
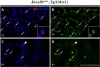
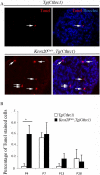
The analysis of the molecular mechanisms involved in the initial interaction between neurons and Schwann cells is a key issue in understanding the myelination process. We recently identified Cthrc1 (Collagen triple helix repeat containing 1) as a gene upregulated in Schwann cells upon interaction with the axon. Cthrc1 encodes a secreted protein previously shown to be involved in migration and proliferation in different cell types. We performed a functional analysis of Cthrc1 in Schwann cells by loss-of- and gain-of-function approaches using RNA interference knockdown in cell culture and a transgenic mouse line that overexpresses the gene. This work establishes that Cthrc1 enhances Schwann cell proliferation but prevents myelination. In particular, time-course analysis of myelin formation intransgenic animals reveals that overexpression of Cthrc1 in Schwann cells leads to a delay in myelin formation with cells maintaining a proliferative state. Our data, therefore, demonstrate that Cthrc1 plays a negative regulatory role, fine-tuning the onset of peripheral myelination.
Figures


Similar articles
-
The atypical Guanine-nucleotide exchange factor, dock7, negatively regulates schwann cell differentiation and myelination.J Neurosci. 2011 Aug 31;31(35):12579-92. doi: 10.1523/JNEUROSCI.2738-11.2011. J Neurosci. 2011. PMID: 21880919 Free PMC article.
-
Cadm3 (Necl-1) interferes with the activation of the PI3 kinase/Akt signaling cascade and inhibits Schwann cell myelination in vitro.Glia. 2016 Dec;64(12):2247-2262. doi: 10.1002/glia.23072. Epub 2016 Sep 23. Glia. 2016. PMID: 27658374 Free PMC article.
-
An In Vitro Model of Charcot-Marie-Tooth Disease Type 4B2 Provides Insight Into the Roles of MTMR13 and MTMR2 in Schwann Cell Myelination.ASN Neuro. 2018 Jan-Dec;10:1759091418803282. doi: 10.1177/1759091418803282. ASN Neuro. 2018. PMID: 30419760 Free PMC article.
-
The Role of CTHRC1 in Regulation of Multiple Signaling and Tumor Progression and Metastasis.Mediators Inflamm. 2020 Aug 12;2020:9578701. doi: 10.1155/2020/9578701. eCollection 2020. Mediators Inflamm. 2020. PMID: 32848510 Free PMC article. Review.
-
Regulation of myelin-specific gene expression. Relevance to CMT1.Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
Cited by
-
Molecular and Regenerative Characterization of Repair and Non-repair Schwann Cells.Cell Mol Neurobiol. 2023 Jul;43(5):2165-2178. doi: 10.1007/s10571-022-01295-4. Epub 2022 Oct 12. Cell Mol Neurobiol. 2023. PMID: 36222946 Free PMC article.
-
Molecular Mechanisms Involved in Schwann Cell Plasticity.Front Mol Neurosci. 2017 Feb 17;10:38. doi: 10.3389/fnmol.2017.00038. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28261057 Free PMC article. Review.
-
SPARCL1 is a novel predictor of tumor recurrence and survival in hilar cholangiocarcinoma.Tumour Biol. 2016 Mar;37(3):4159-67. doi: 10.1007/s13277-015-4206-4. Epub 2015 Oct 21. Tumour Biol. 2016. PMID: 26490986
-
CTHRC1: a key player in colorectal cancer progression and immune evasion.Front Immunol. 2025 Mar 25;16:1579661. doi: 10.3389/fimmu.2025.1579661. eCollection 2025. Front Immunol. 2025. PMID: 40201173 Free PMC article. Review.
-
CTHRC1, a novel gene with multiple functions in physiology, disease and solid tumors (Review).Oncol Lett. 2023 May 3;25(6):266. doi: 10.3892/ol.2023.13852. eCollection 2023 Jun. Oncol Lett. 2023. PMID: 37216164 Free PMC article. Review.
References
-
- Barisic N, Claeys KG, Sirotkovic-Skerlev M, Lofgren A, Nelis E, De Jonghe P, Timmerman V. Charcot-Marie-Tooth disease: a clinico-genetic confrontation. Ann Hum Genet. 2008;72:416–441. - PubMed
-
- Birchmeier C, Nave KA. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia. 2008;56:1491–1497. - PubMed
-
- Blugeon C, Le Crom S, Richard L, Vallat JM, Charnay P, Decker L. Dok4 is involved in Schwann cell myelination and axonal interaction in vitro. Glia in press. 2010 - PubMed
-
- Blugeon C, Le Crom S, Richard L, Vallat JM, Charnay P, Decker L. Dok4 is involved in Schwann cell myelination and axonal interaction in vitro. Glia. 2011;59:351–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

