Novel vaccine development strategies for inducing mucosal immunity
- PMID: 22380827
- PMCID: PMC3315788
- DOI: 10.1586/erv.11.196
Novel vaccine development strategies for inducing mucosal immunity
Abstract
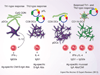
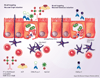
To develop protective immune responses against mucosal pathogens, the delivery route and adjuvants for vaccination are important. The host, however, strives to maintain mucosal homeostasis by responding to mucosal antigens with tolerance, instead of immune activation. Thus, induction of mucosal immunity through vaccination is a rather difficult task, and potent mucosal adjuvants, vectors or other special delivery systems are often used, especially in the elderly. By taking advantage of the common mucosal immune system, the targeting of mucosal dendritic cells and microfold epithelial cells may facilitate the induction of effective mucosal immunity. Thus, novel routes of immunization and antigen delivery systems also show great potential for the development of effective and safe mucosal vaccines against various pathogens. The purpose of this review is to introduce several recent approaches to induce mucosal immunity to vaccines, with an emphasis on mucosal tissue targeting, new immunization routes and delivery systems. Defining the mechanisms of mucosal vaccines is as important as their efficacy and safety, and in this article, examples of recent approaches, which will likely accelerate progress in mucosal vaccine development, are discussed.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was used in the production of this manuscript.
Figures


References
-
-
Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25(30):5467–5484. • Describes the features of the mucosal immune system, including the concept of the common mucosal immune system (CMIS).
-
-
- Lamm ME. Current concepts in mucosal immunity IV. How epithelial transport of IgA antibodies relates to host defense. Am. J. Physiol. 1998;274(4 Pt 1):G614–G617. - PubMed
-
- Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4(6):667–677. - PubMed
-
- Fujihashi K, Boyaka PN, McGhee JR. Host defenses at mucosal surfaces. In: Rich RT, et al., editors. Clinical Immunology. PA, USA: Mosby Elsevier; 2008. pp. 287–304.
-
- Kiyono H, Kunisawa J, McGhee JR, Mestecky J. The mucosal imune system. In: Paul WE, editor. Fundamental Immunology. PA, USA: Lippincott Williams & Wilkins; 2008. pp. 983–1030.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
