ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression
- PMID: 22381410
- PMCID: PMC3635677
- DOI: 10.2967/jnumed.111.094425
ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression
Abstract
We investigated the correlation between metabolic response by (18)F-FDG PET and objective response, glucose transporter type 4 (GLUT4) expression, and KIT/PDGFRA mutation status in patients with gastrointestinal stromal tumor undergoing neoadjuvant imatinib mesylate therapy.
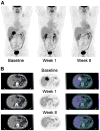
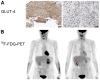
Methods: (18)F-FDG PET was performed at baseline, 1-7 d, and 4 or 8 wk after imatinib mesylate initiation. Best objective response was defined by version 1.0 of the Response Evaluation Criteria in Solid Tumors (RECIST). Mutational analysis and tumor GLUT4 expression by immunohistochemistry were done on tissue obtained at baseline or surgery.
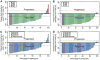
Results: (18)F-FDG PET showed high baseline tumor glycolytic activity (mean SUV(max), 14.2; range, 1.3-53.2), decreasing after 1 wk of imatinib mesylate (mean, 5.5; range, -0.5-47.7, P < 0.001, n = 44), and again before surgery (mean, 3.0; range, -0.5-36.1, P < 0.001, n = 40). At week 1, there were 3 patients with complete metabolic response (CMR), 33 with partial metabolic response (PMR), 6 with stable metabolic disease (SMD), and 2 with progressive metabolic disease (PMD). Before surgery, there were 3 with CMR, 33 with PMR, 4 with SMD, and none with PMD. The best response according to RECIST was 2 with partial response, 36 with stable disease, and 1 with progressive disease (n = 39). Of the patients with a posttreatment decrease in GLUT4 expression, 1 had CMR, 15 had PMR, 2 had SMD, and 1 had PMD at week 1, whereas before surgery 1 patient had CMR, 16 had PMR, 2 had SMD, and none had PMD. Among 27 patients with KIT exon 11 mutations, 1 had CMR, 22 had PMR, 3 had SMD, and 1 had PMD at week 1, whereas 1 had CMR, 22 had PMR, 2 had SMD, and 2 were unknown before surgery; among 4 patients with a wild-type genotype, 2 had PMR and 2 SMD at week 1, whereas 1 had CMR, 2 had PMR, and 1 had SMD before surgery.
Conclusion: After imatinib mesylate initiation, metabolic response by (18)F-FDG PET was documented earlier (1-7 d) and was of much greater magnitude (36/44) than that documented by RECIST (2/39). Immunohistochemistry data suggest that GLUT4 may play a role in (18)F-FDG uptake in gastrointestinal stromal tumor, GLUT4 levels decrease after imatinib mesylate therapy in most patients with PMR, and the biologic action of imatinib mesylate interacts with glycolysis and GLUT4 expression. A greater than 85% metabolic response was observed as early as days 1-7 in patients with exon 11 mutations.
Figures
Similar articles
-
Metabolic response by FDG-PET to imatinib correlates with exon 11 KIT mutation and predicts outcome in patients with mucosal melanoma.Cancer Imaging. 2014 Nov 12;14(1):30. doi: 10.1186/s40644-014-0030-0. Cancer Imaging. 2014. PMID: 25609545 Free PMC article. Clinical Trial.
-
Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor.J Clin Oncol. 2009 Jan 20;27(3):439-45. doi: 10.1200/JCO.2008.17.2742. Epub 2008 Dec 8. J Clin Oncol. 2009. PMID: 19064982 Clinical Trial.
-
[18F-FDG PET/CT in response evaluation of gastrointestinal stromal tumours treated with imatinib].Rev Esp Med Nucl. 2008 May-Jun;27(3):168-75. Rev Esp Med Nucl. 2008. PMID: 18570858 Clinical Trial. Spanish.
-
The role of KIT in the management of patients with gastrointestinal stromal tumors.Hum Pathol. 2007 May;38(5):679-87. doi: 10.1016/j.humpath.2007.03.001. Hum Pathol. 2007. PMID: 17437861 Review.
-
Gastrointestinal stromal tumors: who should get imatinib and for how long?Adv Surg. 2014;48(1):165-83. doi: 10.1016/j.yasu.2014.05.014. Adv Surg. 2014. PMID: 25293614 Free PMC article. Review.
Cited by
-
Novel Insights into the Treatment of Imatinib-Resistant Gastrointestinal Stromal Tumors.Target Oncol. 2017 Jun;12(3):277-288. doi: 10.1007/s11523-017-0490-9. Target Oncol. 2017. PMID: 28478525 Review.
-
The predictive value of preoperative 18F-fluorodeoxyglucose PET for postoperative recurrence in patients with localized primary gastrointestinal stromal tumour.Eur Radiol. 2016 Dec;26(12):4664-4674. doi: 10.1007/s00330-016-4242-5. Epub 2016 Feb 6. Eur Radiol. 2016. PMID: 26852217
-
State of the Art in the Treatment of Gastrointestinal Stromal Tumors.Gastrointest Tumors. 2014 May;1(4):221-36. doi: 10.1159/000380788. Epub 2015 Apr 21. Gastrointest Tumors. 2014. PMID: 26672673 Free PMC article. Review.
-
Pathological Complete Response After Neoadjuvant Imatinib in a Recurrent Duodenal Gastrointestinal Stromal Tumor (GIST).Cureus. 2024 Jul 16;16(7):e64669. doi: 10.7759/cureus.64669. eCollection 2024 Jul. Cureus. 2024. PMID: 39149625 Free PMC article.
-
Beyond standard therapy: drugs under investigation for the treatment of gastrointestinal stromal tumor.Expert Opin Investig Drugs. 2015;24(8):1045-58. doi: 10.1517/13543784.2015.1046594. Epub 2015 Jun 22. Expert Opin Investig Drugs. 2015. PMID: 26098203 Free PMC article. Review.
References
-
- Miettinen M. Gastrointestinal stromal tumors: definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. - PubMed
-
- Hirota S, Isozaki K, Nishida T, Kitamura Y. Effects of loss-of-function and gain-of-function mutations of c-kit on the gastrointestinal tract. J Gastroenterol. 2000;35(suppl 12):75–79. - PubMed
-
- Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. - PubMed
-
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. - PubMed
-
- Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
