Amelioration of Duchenne muscular dystrophy in mdx mice by elimination of matrix-associated fibrin-driven inflammation coupled to the αMβ2 leukocyte integrin receptor
- PMID: 22381526
- PMCID: PMC3315206
- DOI: 10.1093/hmg/dds012
Amelioration of Duchenne muscular dystrophy in mdx mice by elimination of matrix-associated fibrin-driven inflammation coupled to the αMβ2 leukocyte integrin receptor
Abstract
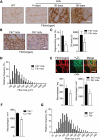
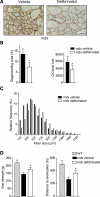
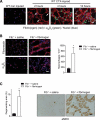
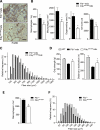
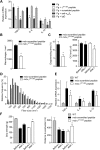
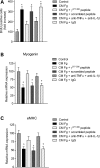
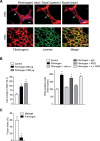
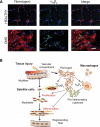
In Duchenne muscular dystrophy (DMD), a persistently altered and reorganizing extracellular matrix (ECM) within inflamed muscle promotes damage and dysfunction. However, the molecular determinants of the ECM that mediate inflammatory changes and faulty tissue reorganization remain poorly defined. Here, we show that fibrin deposition is a conspicuous consequence of muscle-vascular damage in dystrophic muscles of DMD patients and mdx mice and that elimination of fibrin(ogen) attenuated dystrophy progression in mdx mice. These benefits appear to be tied to: (i) a decrease in leukocyte integrin α(M)β(2)-mediated proinflammatory programs, thereby attenuating counterproductive inflammation and muscle degeneration; and (ii) a release of satellite cells from persistent inhibitory signals, thereby promoting regeneration. Remarkably, Fib-gamma(390-396A) (Fibγ(390-396A)) mice expressing a mutant form of fibrinogen with normal clotting function, but lacking the α(M)β(2) binding motif, ameliorated dystrophic pathology. Delivery of a fibrinogen/α(M)β(2) blocking peptide was similarly beneficial. Conversely, intramuscular fibrinogen delivery sufficed to induce inflammation and degeneration in fibrinogen-null mice. Thus, local fibrin(ogen) deposition drives dystrophic muscle inflammation and dysfunction, and disruption of fibrin(ogen)-α(M)β(2) interactions may provide a novel strategy for DMD treatment.
Figures
Similar articles
-
Fibrin(ogen) drives repair after acetaminophen-induced liver injury via leukocyte αMβ2 integrin-dependent upregulation of Mmp12.J Hepatol. 2017 Apr;66(4):787-797. doi: 10.1016/j.jhep.2016.12.004. Epub 2016 Dec 10. J Hepatol. 2017. PMID: 27965156 Free PMC article.
-
miR-146a deficiency does not aggravate muscular dystrophy in mdx mice.Skelet Muscle. 2019 Aug 14;9(1):22. doi: 10.1186/s13395-019-0207-0. Skelet Muscle. 2019. PMID: 31412923 Free PMC article.
-
Delayed bone regeneration is linked to chronic inflammation in murine muscular dystrophy.J Bone Miner Res. 2014 Feb;29(2):304-15. doi: 10.1002/jbmr.2038. J Bone Miner Res. 2014. PMID: 23857747 Free PMC article.
-
Fibrin(ogen)-alpha M beta 2 interactions regulate leukocyte function and innate immunity in vivo.Exp Biol Med (Maywood). 2004 Dec;229(11):1105-10. doi: 10.1177/153537020422901104. Exp Biol Med (Maywood). 2004. PMID: 15564436 Review.
-
Satellite Cells in Muscular Dystrophy - Lost in Polarity.Trends Mol Med. 2016 Jun;22(6):479-496. doi: 10.1016/j.molmed.2016.04.002. Epub 2016 May 5. Trends Mol Med. 2016. PMID: 27161598 Free PMC article. Review.
Cited by
-
The Promiscuous Profile of Complement Receptor 3 in Ligand Binding, Immune Modulation, and Pathophysiology.Front Immunol. 2021 Apr 29;12:662164. doi: 10.3389/fimmu.2021.662164. eCollection 2021. Front Immunol. 2021. PMID: 33995387 Free PMC article. Review.
-
Loss of full-length dystrophin expression results in major cell-autonomous abnormalities in proliferating myoblasts.Elife. 2022 Sep 27;11:e75521. doi: 10.7554/eLife.75521. Elife. 2022. PMID: 36164827 Free PMC article.
-
Fibrin accumulation secondary to loss of plasmin-mediated fibrinolysis drives inflammatory osteoporosis in mice.Arthritis Rheumatol. 2014 Aug;66(8):2222-33. doi: 10.1002/art.38639. Arthritis Rheumatol. 2014. PMID: 24664548 Free PMC article.
-
From innate to adaptive immune response in muscular dystrophies and skeletal muscle regeneration: the role of lymphocytes.Biomed Res Int. 2014;2014:438675. doi: 10.1155/2014/438675. Epub 2014 Jun 16. Biomed Res Int. 2014. PMID: 25028653 Free PMC article. Review.
-
Geriatric muscle stem cells switch reversible quiescence into senescence.Nature. 2014 Feb 20;506(7488):316-21. doi: 10.1038/nature13013. Epub 2014 Feb 12. Nature. 2014. PMID: 24522534
References
-
- Campbell K.P. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi:10.1016/0092-8674(95)90344-5. - DOI - PubMed
-
- Sicinski P., Geng Y., Ryder-Cook A.S., Barnard E.A., Darlison M.G., Barnard P.J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi:10.1126/science.2662404. - DOI - PubMed
-
- Durbeej M., Campbell K.P. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr. Opin. Genet. Dev. 2002;12:349–361. doi:10.1016/S0959-437X(02)00309-X. - DOI - PubMed
-
- Tidball J.G. Mechanical signal transduction in skeletal muscle growth and adaptation. J. Appl. Physiol. 2005;98:1900–1908. doi:10.1152/japplphysiol.01178.2004. - DOI - PubMed
-
- Stedman H.H., Sweeney H.L., Shrager J.B., Maguire H.C., Panettieri R.A., Petrof B., Narusawa M., Leferovich J.M., Sladky J.T., Kelly A.M. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi:10.1038/352536a0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

