Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits?
- PMID: 22382359
- PMCID: PMC3501966
- DOI: 10.1093/brain/aws023
Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits?
Abstract
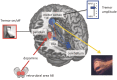
Tremor in Parkinson's disease has several mysterious features. Clinically, tremor is seen in only three out of four patients with Parkinson's disease, and tremor-dominant patients generally follow a more benign disease course than non-tremor patients. Pathophysiologically, tremor is linked to altered activity in not one, but two distinct circuits: the basal ganglia, which are primarily affected by dopamine depletion in Parkinson's disease, and the cerebello-thalamo-cortical circuit, which is also involved in many other tremors. The purpose of this review is to integrate these clinical and pathophysiological features of tremor in Parkinson's disease. We first describe clinical and pathological differences between tremor-dominant and non-tremor Parkinson's disease subtypes, and then summarize recent studies on the pathophysiology of tremor. We also discuss a newly proposed 'dimmer-switch model' that explains tremor as resulting from the combined actions of two circuits: the basal ganglia that trigger tremor episodes and the cerebello-thalamo-cortical circuit that produces the tremor. Finally, we address several important open questions: why resting tremor stops during voluntary movements, why it has a variable response to dopaminergic treatment, why it indicates a benign Parkinson's disease subtype and why its expression decreases with disease progression.
Figures

References
-
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–92. - PubMed
-
- Abdo WF, van de Warrenburg BP, Burn DJ, Quinn NP, Bloem BR. The clinical approach to movement disorders. Nat Rev Neurol. 2010;6:29–37. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75. - PubMed
-
- Albo Z, Di Prisco GV, Chen YH, Rangarajan G, Truccolo W, Feng JF, Vertes RP, Ding MZ. Is partial coherence a viable technique for identifying generators of neural oscillations? Biol Cybernet. 2004;90:318–26. - PubMed
-
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord. 2006;21:1123–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

