Understanding the genetic and molecular pathogenesis of Friedreich's ataxia through animal and cellular models
- PMID: 22382366
- PMCID: PMC3291638
- DOI: 10.1242/dmm.008706
Understanding the genetic and molecular pathogenesis of Friedreich's ataxia through animal and cellular models
Abstract
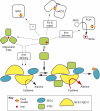
In 1996, a link was identified between Friedreich's ataxia (FRDA), the most common inherited ataxia in men, and alterations in the gene encoding frataxin (FXN). Initial studies revealed that the disease is caused by a unique, most frequently biallelic, expansion of the GAA sequence in intron 1 of FXN. Since the identification of this link, there has been tremendous progress in understanding frataxin function and the mechanism of FRDA pathology, as well as in developing diagnostics and therapeutic approaches for the disease. These advances were the subject of the 4th International Friedreich's Ataxia Conference held on 5th-7th May in the Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France. More than 200 scientists gathered from all over the world to present the results of research spanning all areas of investigation into FRDA (including clinical aspects, FRDA pathogenesis, genetics and epigenetics of the disease, development of new models of FRDA, and drug discovery). This review provides an update on the understanding of frataxin function, developments of animal and cellular models of the disease, and recent advances in trying to uncover potential molecules for therapy.
Figures
References
-
- Adinolfi S., Iannuzzi C., Prischi F., Pastore C., Iametti S., Martin S. R., Bonomi F., Pastore A. (2009). Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat. Struct. Mol. Biol. 16, 390–396 - PubMed
-
- Al-Mahdawi S., Pinto R. M., Varshney D., Lawrence L., Lowrie M. B., Hughes S., Webster Z., Blake J., Cooper J. M., King R., et al. (2006). GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics 88, 580–590 - PMC - PubMed
-
- Al-Mahdawi S., Pinto R. M., Ismail O., Varshney D., Lymperi S., Sandi C., Trabzuni D., Pook M. (2008). The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum. Mol. Genet. 17, 735–746 - PubMed
-
- Anderson P. R., Kirby K., Hilliker A. J., Phillips J. P. (2005). RNAi-mediated suppression of the mitochondrial iron chaperone, frataxin, in Drosophila. Hum. Mol. Genet. 14, 3397–3405 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

