Adult brainstem gliomas
- PMID: 22382458
- PMCID: PMC3316925
- DOI: 10.1634/theoncologist.2011-0335
Adult brainstem gliomas
Abstract
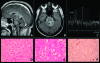
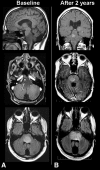
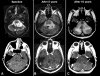
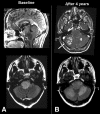
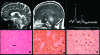
Brainstem gliomas are uncommon in adults and account for only 1%-2% of intracranial gliomas. They represent a heterogeneous group of tumors that differ from those found in their pediatric counterparts. In adults, a low-grade phenotype predominates, which is a feature that likely explains their better prognosis compared to that in children. Because biopsies are rarely performed, classifications based on the radiological aspect of magnetic resonance imaging results have been proposed to establish treatment strategies and to determine outcomes: (a) diffuse intrinsic low-grade, (b) enhancing malignant glioma, (c) focal tectal gliomas, and (d) exophytic gliomas. Despite significant advances in neuroradiology techniques, a purely radiological classification remains imperfect in the absence of a histological diagnosis. Whereas a biopsy may often be reasonably avoided in the diffuse nonenhancing forms, obtaining histological proof seems necessary in many contrast-enhanced brainstem lesions because of the wide variety of differential diagnoses in adults. Conventional radiotherapy is the standard treatment for diffuse intrinsic low-grade brainstem gliomas in adults (the median survival is 5 years). In malignant brainstem gliomas, radiotherapy is the standard treatment. However, the possible benefit of combined radiotherapy and chemotherapy (temozolomide or other agents) has not been thoroughly evaluated in adults. The role of anti-angiogenic therapies in brainstem gliomas remains to be defined. A better understanding of the biology of these tumors is of primary importance for identifying homogeneous subgroups and for improving therapy options and outcomes.
Conflict of interest statement
Section Editor:
Reviewer “A”: Procure (C/A); UpToDate (H); NCI (RF).
Figures
Similar articles
-
Brainstem gliomas in adults: prognostic factors and classification.Brain. 2001 Dec;124(Pt 12):2528-39. doi: 10.1093/brain/124.12.2528. Brain. 2001. PMID: 11701605
-
Imaging of adult brainstem gliomas.Eur J Radiol. 2015 Apr;84(4):709-20. doi: 10.1016/j.ejrad.2014.12.025. Epub 2015 Jan 7. Eur J Radiol. 2015. PMID: 25641008 Review.
-
Pediatric high-grade gliomas and diffuse intrinsic pontine gliomas.J Child Neurol. 2009 Nov;24(11):1409-17. doi: 10.1177/0883073809338960. Epub 2009 Jul 28. J Child Neurol. 2009. PMID: 19638636 Review.
-
The Role of Advanced MRI Sequences in the Diagnosis and Follow-Up of Adult Brainstem Gliomas: A Neuroradiological Review.Tomography. 2023 Aug 18;9(4):1526-1537. doi: 10.3390/tomography9040122. Tomography. 2023. PMID: 37624115 Free PMC article. Review.
-
Adult brainstem gliomas.Cancer. 2016 Sep 15;122(18):2799-809. doi: 10.1002/cncr.29920. Epub 2016 Jun 21. Cancer. 2016. PMID: 27327773 Review.
Cited by
-
Adult brainstem gliomas: Correlation of clinical and molecular features.J Neurol Sci. 2015;353(1-2):92-7. doi: 10.1016/j.jns.2015.04.014. Epub 2015 Apr 18. J Neurol Sci. 2015. PMID: 25934342 Free PMC article.
-
An uncommon presentation of early brainstem high-grade glioma in a 33-year-old male: A case study and review of literature.Int J Surg Case Rep. 2024 Jan;114:109152. doi: 10.1016/j.ijscr.2023.109152. Epub 2023 Dec 13. Int J Surg Case Rep. 2024. PMID: 38141508 Free PMC article.
-
Clinical characteristics and prognostic factors of adult brainstem gliomas: A retrospective analysis of histologically-proven 40 cases.Medicine (Baltimore). 2024 May 3;103(18):e37910. doi: 10.1097/MD.0000000000037910. Medicine (Baltimore). 2024. PMID: 38701282 Free PMC article.
-
Brainstem glioma: Prediction of histopathologic grade based on conventional MR imaging.Neuroradiol J. 2018 Feb;31(1):10-17. doi: 10.1177/1971400917743099. Epub 2017 Nov 17. Neuroradiol J. 2018. PMID: 29148317 Free PMC article.
-
Rotating Gamma System Irradiation: A Promising Treatment for Low-grade Brainstem Gliomas.In Vivo. 2017 Sep-Oct;31(5):957-960. doi: 10.21873/invivo.11153. In Vivo. 2017. PMID: 28882965 Free PMC article.
References
-
- Barkovich AJ, Krischer J, Kun LE, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16:73–83. - PubMed
-
- Donaldson SS, Laningham F, Fisher P, et al. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24:1266–1272. - PubMed
-
- Fischbein NJ, Prados MD, Wara W, et al. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996;24:9–23. - PubMed
-
- Guillamo JS, Monjour A, Taillandier L, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001;124:2528–2539. - PubMed
-
- Landolfi JC, Thaler HT, DeAngelis LM. Adult brainstem gliomas. Neurology. 1998;51:1136–1139. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

