Clinical, pharmacokinetic and pharmacodynamic evaluations of metronomic UFT and cyclophosphamide plus celecoxib in patients with advanced refractory gastrointestinal cancers
- PMID: 22382585
- PMCID: PMC3338912
- DOI: 10.1007/s10456-012-9260-6
Clinical, pharmacokinetic and pharmacodynamic evaluations of metronomic UFT and cyclophosphamide plus celecoxib in patients with advanced refractory gastrointestinal cancers
Abstract
Aims: To evaluate UFT and cyclophosphamide (CTX) based metronomic chemotherapy plus celecoxib (CXB) for the treatment of patients with heavily pre-treated advanced gastrointestinal malignancies.
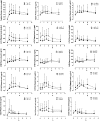
Methods: Thirty-eight patients received 500 mg/mq(2) CTX i.v bolus on day 1 and, from day 2, 50 mg/day CTX p.o. plus 100 mg/twice a day UFT p.o. and 200 mg/twice a day CXB p.o. Tegafur, 5-FU, 5-FUH(2), GHB and uracil pharmacokinetics were assessed. Plasma vascular endothelial growth factor (VEGF), soluble VE-cadherin (sVE-C) and thrombospondin-1 (TSP-1) levels were detected by ELISA and real-time PCR of CD133 gene expression on peripheral blood mononuclear cell was also performed.
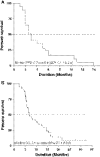
Results: Seventeen patients (45%) obtained stable disease (SD) with a median duration of 5.8 ms (range, 4.2-7.4). Median progression free survival (PFS) and overall survival (OS) were 2.7 ms (95% CI, 1.6-3.9 ms) and 7.1 ms (95% CI, 4.3-9.9 ms), respectively. No toxicities of grade >1 were observed. Pharmacokinetics of 27 patients (13/14, SD/progressive disease, PD) after the first treatment of UFT revealed that 5-FU AUC and C(max) values greater than 1.313 h × μg/ml and 0.501 μg/ml, respectively, were statistically correlated with stabilization of disease and prolonged PFS/OS. VEGF and sVE-C plasma levels were greater in the PD group when compared to SD group. CD133 expression increased only in the PD patients.
Conclusion: Metronomic UFT and CTX with CXB in heavily pre-treated gastrointestinal patients were well tolerated and associated with interesting activity. Potential predictive pharmacokinetic parameters and pharmacodynamic biomarkers have been found.
Figures

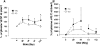
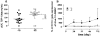
References
-
- Bocci G, Tuccori M, Emmenegger U, Liguori V, Falcone A, Kerbel RS, Del Tacca M. Cyclophosphamide-methotrexate ‘metronomic’ chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol. 2005;16(8):1243–1252. doi: 10.1093/annonc/mdi240. - DOI - PubMed
-
- Fioravanti A, Canu B, Ali G, Orlandi P, Allegrini G, Di Desidero T, Emmenegger U, Fontanini G, Danesi R, Del Tacca M, Falcone A, Bocci G. Metronomic 5-fluorouracil, oxaliplatin and irinotecan in colorectal cancer. Eur J Pharmacol. 2009;619(1–3):8–14. doi: 10.1016/j.ejphar.2009.08.020. - DOI - PubMed
-
- Rapisarda A, Zalek J, Hollingshead M, Braunschweig T, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, Hewitt SM, Shoemaker RH, Melillo G. Schedule-dependent inhibition of hypoxia-inducible factor-1 protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Res. 2004;64(19):6845–6848. doi: 10.1158/0008-5472.CAN-04-2116. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

