Tumor dosimetry using [124I]m-iodobenzylguanidine microPET/CT for [131I]m-iodobenzylguanidine treatment of neuroblastoma in a murine xenograft model
- PMID: 22382618
- PMCID: PMC3369020
- DOI: 10.1007/s11307-012-0552-4
Tumor dosimetry using [124I]m-iodobenzylguanidine microPET/CT for [131I]m-iodobenzylguanidine treatment of neuroblastoma in a murine xenograft model
Abstract
Purpose: [(124)I]m-iodobenzylguanidine ((124)I-mIBG) provides a quantitative tool for pretherapy tumor imaging and dosimetry when performed before [(131)I]m-iodobenzylguanidine ((131)I-mIBG) targeted radionuclide therapy of neuroblastoma. (124)I (T (1/2) = 4.2 days) has a comparable half-life to that of (131)I (T (1/2) = 8.02 days) and can be imaged by positron emission tomography (PET) for accurate quantification of the radiotracer distribution. We estimated expected radiation dose in tumors from (131)I-mIBG therapy using (124)I-mIBG microPET/CT imaging data in a murine xenograft model of neuroblastoma transduced to express high levels of the human norepinephrine transporter (hNET).
Procedures: In order to enhance mIBG uptake for in vivo imaging and therapy, NB 1691-luciferase (NB1691) human neuroblastoma cells were engineered to express high levels of hNET protein by lentiviral transduction (NB1691-hNET). Both NB1691 and NB1691-hNET cells were implanted subcutaneously and into renal capsules in athymic mice. (124)I-mIBG (4.2-6.5 MBq) was administered intravenously for microPET/CT imaging at 5 time points over 95 h (0.5, 3-5, 24, 48, and 93-95 h median time points). In vivo biodistribution data in normal organs, tumors, and whole-body were collected from reconstructed PET images corrected for photon attenuation using the CT-based attenuation map. Organ and tumor dosimetry were determined for (124)I-mIBG. Dose estimates for (131)I-mIBG were made, assuming the same in vivo biodistribution as (124)I-mIBG.
Results: All NB1691-hNET tumors had significant uptake and retention of (124)I-mIBG, whereas unmodified NB1691 tumors did not demonstrate quantifiable mIBG uptake in vivo, despite in vitro uptake. (124)I-mIBG with microPET/CT provided an accurate three-dimensional tool for estimating the radiation dose that would be delivered with (131)I-mIBG therapy. For example, in our model system, we estimated that the administration of (131)I-mIBG in the range of 52.8-206 MBq would deliver 20 Gy to tumors.
Conclusions: The overexpression of hNET was found to be critical for (124)I-mIBG uptake and retention in vivo. The quantitative (124)I-mIBG PET/CT is a promising new tool to predict tumor radiation doses with (131)I-mIBG therapy of neuroblastoma. This methodology may be applied to tumor dosimetry of (131)I-mIBG therapy in human subjects using (124)I-mIBG pretherapy PET/CT data.
Conflict of interest statement
Figures
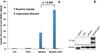


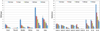
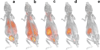
References
-
- Mueller S, Matthay KK. Neuroblastoma: biology and staging. Curr Oncol Rep. 2009;11:431–438. - PubMed
-
- Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. - PubMed
-
- Short JH, Darby TD. Sympathetic nervous system blocking agents. 3. Derivatives of benzylguanidine. J Med Chem. 1967;10:833–840. - PubMed
-
- Wieland DM, Brown LE, Tobes MC, Rogers WL, Marsh DD, Mangner TJ, Swanson DP, Beierwaltes WH. Imaging the primate adrenal medulla with [123I] and [131I] meta-iodobenzylguanidine: concise communication. J Nucl Med. 1981;22:358–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

