'Hearts and bones': the ups and downs of 'plasticity' in stem cell biology
- PMID: 22383126
- PMCID: PMC3403293
- DOI: 10.1002/emmm.201200220
'Hearts and bones': the ups and downs of 'plasticity' in stem cell biology
Abstract
More than a decade ago, 'plasticity' suddenly became a 'fashionable' topic with overemphasized implications for regenerative medicine. The concept of 'plasticity' is supported by old transplantation work, at least for embryonic cells, and metaplasia is a classic example of plasticity observed in patients. Nevertheless, the publication of a series of papers showing rare conversion of a given cell type into another unrelated cell raised the possibility of using any unaffected tissue to create at will new cells to replace a different failing tissue or organ. This resulted in disingenuous interpretations and a reason not to fund anymore research on embryonic stem cells (ESc). Moreover, many papers on plasticity were difficult to reproduce and thus questioned; raising issues about plasticity as a technical artefact or a consequence of rare spontaneous cells fusion. More recently, reprogramming adult differentiated cells to a pluripotent state (iPS) became possible, and later, one type of differentiated cell could be directly reprogrammed into another (e.g. fibroblasts into neurons) without reverting to pluripotency. Although the latter results from different and more robust experimental protocols, these phenomena also exemplify 'plasticity'. In this review, we want to place 'plasticity' in a historical perspective still taking into account ethical and political implications.
Copyright © 2012 EMBO Molecular Medicine.
Figures

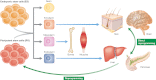
Nuclear transfer into an anucleated oocyte reprograms a somatic nucleus, thus generating a blastocyst from which ES cells can be derived.
Cell fusion exposes two different nuclei to the same cytoplasm, and one nucleus may impose its transcriptional program (red) over the other (blue).
Transfer of specific transcription factors may reprogram a somatic cell (yellow) to a pluripotent (iPS) or to another differentiated cell type (red).
Transplantation of a genetically labeled (blue nucleus) differentiated cell (red) to a different tissue (yellow) may activate that developmental program in the transplanted cell.
References
-
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. - PubMed
-
- Avila D. The present standing of the human embryo in U.S. law. Natl Cathol Bioeth Q. 2001;1:203–226. - PubMed
-
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

