A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a position paper
- PMID: 22383228
- PMCID: PMC3389500
- DOI: 10.1177/1352458512438454
A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a position paper
Abstract
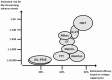
Background: Haematopoietic stem cell transplantation (HSCT) has been tried in the last 15 years as a therapeutic option in patients with poor-prognosis autoimmune disease who do not respond to conventional treatments. Worldwide, more than 600 patients with multiple sclerosis (MS) have been treated with HSCT, most of them having been recruited in small, single-centre, phase 1-2 uncontrolled trials. Clinical and magnetic resonance imaging outcomes from case series reports or Registry-based analyses suggest that a major response is achieved in most patients; quality and duration of response are better in patients transplanted during the relapsing-remitting phase than in those in the secondary progressive stage.
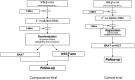
Objectives: An interdisciplinary group of neurologists and haematologists has been formed, following two international meetings supported by the European and American Blood and Marrow Transplantation Societies, for the purpose of discussing a controlled clinical trial, to be designed within the new scenarios of evolving MS treatments.
Conclusions: Objectives of the trial, patient selection, transplant technology and outcome assessment were extensively discussed. The outcome of this process is summarized in the present paper, with the goal of establishing the background and advancing the development of a prospective, randomized, controlled multicentre trial to assess the clinical efficacy of HSCT for the treatment of highly active MS.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Comment in
-
Autologous hematopoietic stem cell transplantation for multiple sclerosis--if confused or hesitant, remember: 'treat with standard immune suppressive drugs and if no inflammation, no response'.Mult Scler. 2012 Jun;18(6):772-5. doi: 10.1177/1352458512442993. Mult Scler. 2012. PMID: 22619224 No abstract available.
References
-
- Niino M, Sasaki H. Update on the treatment options for multiple sclerosis. Expert Rev Clin Immunol 2010; 6: 77-88 - PubMed
-
- Ljungman P, Bregni M, Brune M, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant 2010; 45: 219-234 - PubMed
-
- Saccardi R, Di Gioia M, Bosi A. Haematopoietic stem cell transplantation for autoimmune disorders. Curr Opin Hematol 2008; 15: 594-600 - PubMed
-
- van Bekkum DW. Stem cell transplantation in experimental models of autoimmune disease. J Clin Immunol 2000; 20: 10-16 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

