Multimodality MR imaging findings of low-grade brain edema in hepatic encephalopathy
- PMID: 22383235
- PMCID: PMC7964470
- DOI: 10.3174/ajnr.A2968
Multimodality MR imaging findings of low-grade brain edema in hepatic encephalopathy
Abstract
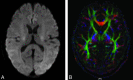
HE is a neuropsychiatric syndrome that develops in patients with severe liver diseases, with portosystemic shunt surgery in the form of diffuse mild brain edema. It is also associated with functional changes, such as those in attention and the DMN. MR imaging offers a range of capabilities for assessing the low-grade brain edema and its functional changes in brain affected by HE; therefore, it provides the opportunity to uncover the pathophysiologic mechanisms of HE. This article will review our current understanding of the pathophysiology of low-grade brain edema and will outline the role of structural MR imaging, MTR, DWI, DTI, and MR spectroscopy in the detection of low-grade brain edema, and the role of BOLD fMRI in the exploration of the related functional changes. A perspective of the study in this area will also be provided.
Figures





References
-
- Córdoba J. New assessment of hepatic encephalopathy. J Hepatol 2011;54:1030–40 - PubMed
-
- Poveda MJ, Bernabeu A, Concepción L, et al. . Brain edema dynamics in patients with overt hepatic encephalopathy: a magnetic resonance imaging study. Neuroimage 2010;52:481–87 - PubMed
-
- McPhail MJ, Taylor-Robinson SD. The role of magnetic resonance imaging and spectroscopy in hepatic encephalopathy. Metab Brain Dis 2010;25:65–72 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
