Investigations on the 4-quinolone-3-carboxylic acid motif part 5: modulation of the physicochemical profile of a set of potent and selective cannabinoid-2 receptor ligands through a bioisosteric approach
- PMID: 22383251
- PMCID: PMC3516921
- DOI: 10.1002/cmdc.201100573
Investigations on the 4-quinolone-3-carboxylic acid motif part 5: modulation of the physicochemical profile of a set of potent and selective cannabinoid-2 receptor ligands through a bioisosteric approach
Abstract
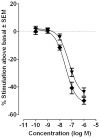
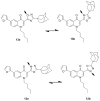
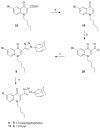
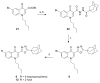
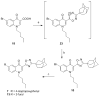
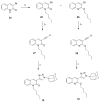
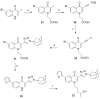
Three heterocyclic systems were selected as potential bioisosteres of the amide linker for a series of 1,6-disubstituted-4-quinolone-3-carboxamides, which are potent and selective CB2 ligands that exhibit poor water solubility, with the aim of improving their physicochemical profile and also of clarifying properties of importance for amide bond mimicry. Among the newly synthesized compounds, a 1,2,3-triazole derivative (1-(adamantan-1-yl)-4-[6-(furan-2-yl)-1,4-dihydro-4-oxo-1-pentylquinolin-3-yl]-1H-1,2,3-triazole) emerged as the most promising in terms of both physicochemical and pharmacodynamic properties. When assayed in vitro, this derivative exhibited inverse agonist activity, whereas, in the formalin test in mice, it produced analgesic effects antagonized by a well-established inverse agonist. Metabolic studies allowed the identification of a side chain hydroxylated derivative as its only metabolite, which, in its racemic form, still showed appreciable CB2 selectivity, but was 150-fold less potent than the parent compound.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
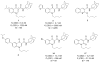
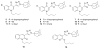
Similar articles
-
Investigations on the 4-quinolone-3-carboxylic acid motif. 3. Synthesis, structure-affinity relationships, and pharmacological characterization of 6-substituted 4-quinolone-3-carboxamides as highly selective cannabinoid-2 receptor ligands.J Med Chem. 2010 Aug 26;53(16):5915-28. doi: 10.1021/jm100123x. J Med Chem. 2010. PMID: 20718492
-
In vitro and in vivo pharmacological characterization of two novel selective cannabinoid CB(2) receptor inverse agonists.Pharmacol Res. 2010 Apr;61(4):349-54. doi: 10.1016/j.phrs.2009.11.011. Epub 2009 Dec 2. Pharmacol Res. 2010. PMID: 19961936
-
Investigations on the 4-quinolone-3-carboxylic acid motif. 6. Synthesis and pharmacological evaluation of 7-substituted quinolone-3-carboxamide derivatives as high affinity ligands for cannabinoid receptors.Eur J Med Chem. 2012 Dec;58:30-43. doi: 10.1016/j.ejmech.2012.09.035. Epub 2012 Oct 3. Eur J Med Chem. 2012. PMID: 23085772
-
Investigations on the 4-Quinolone-3-carboxylic Acid Motif. 7. Synthesis and Pharmacological Evaluation of 4-Quinolone-3-carboxamides and 4-Hydroxy-2-quinolone-3-carboxamides as High Affinity Cannabinoid Receptor 2 (CB2R) Ligands with Improved Aqueous Solubility.J Med Chem. 2016 Feb 11;59(3):1052-67. doi: 10.1021/acs.jmedchem.5b01559. Epub 2016 Jan 26. J Med Chem. 2016. PMID: 26756097
-
The 4-quinolone-3-carboxylic acid motif as a multivalent scaffold in medicinal chemistry.Curr Med Chem. 2009;16(14):1746-67. doi: 10.2174/092986709788186156. Curr Med Chem. 2009. PMID: 19442143 Review.
Cited by
-
Catalyst-free assembly of giant tris(heteroaryl)methanes: synthesis of novel pharmacophoric triads and model sterically crowded tris(heteroaryl/aryl)methyl cation salts.Beilstein J Org Chem. 2019 Mar 12;15:642-654. doi: 10.3762/bjoc.15.60. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 30931006 Free PMC article.
-
Systematic Modification of the Substitution Pattern of the 7-Hydroxy-5-oxopyrazolo[4,3-b]pyridine-6-carboxamide Scaffold Enabled the Discovery of New Ligands with High Affinity and Selectivity for the Cannabinoid Type 2 Receptor.Molecules. 2023 Jun 24;28(13):4958. doi: 10.3390/molecules28134958. Molecules. 2023. PMID: 37446625 Free PMC article.
-
Structure-Activity Relationship of Cannabis Derived Compounds for the Treatment of Neuronal Activity-Related Diseases.Molecules. 2018 Jun 25;23(7):1526. doi: 10.3390/molecules23071526. Molecules. 2018. PMID: 29941830 Free PMC article. Review.
-
Evaluation of 1,2,3-Triazoles as Amide Bioisosteres In Cystic Fibrosis Transmembrane Conductance Regulator Modulators VX-770 and VX-809.Chemistry. 2019 Mar 7;25(14):3662-3674. doi: 10.1002/chem.201805919. Epub 2019 Feb 11. Chemistry. 2019. PMID: 30650214 Free PMC article.
-
N-palmitoyl-D-glucosamine, a Natural Monosaccharide-Based Glycolipid, Inhibits TLR4 and Prevents LPS-Induced Inflammation and Neuropathic Pain in Mice.Int J Mol Sci. 2021 Feb 2;22(3):1491. doi: 10.3390/ijms22031491. Int J Mol Sci. 2021. PMID: 33540826 Free PMC article.
References
-
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Nature. 1990;346:561–564. - PubMed
-
- Munro S, Thomas KL, Abu-Shaar M. Nature. 1993;365:61–65. - PubMed
-
- Pertwee RG. In: Cannabinoids. Pertwee R, editor. Vol. 168. New York: Springer; 2005. pp. 1–51. - PubMed
-
- Pertwee RG, Ross RA. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

