Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development
- PMID: 22383462
- PMCID: PMC5417137
- DOI: 10.1210/me.2011-1362
Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development
Abstract
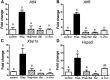
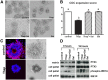
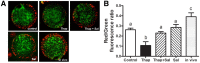
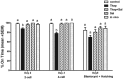
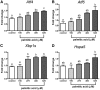
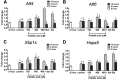
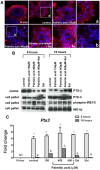
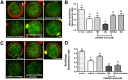
Fatty acids such as palmitic acid at high levels are known to induce endoplasmic reticulum (ER) stress and lipotoxicity in numerous cell types and thereby contribute to cellular dysfunctions in obesity. To understand the impact of high fatty acids on oocytes, ER stress and lipotoxicity were induced in mouse cumulus-oocyte complexes during in vitro maturation using the ER Ca(2+) channel blocker thapsigargin or high physiological levels of palmitic acid; both of which significantly induced ER stress marker genes (Atf4, Atf6, Xbp1s, and Hspa5) and inositol-requiring protein-1α phosphorylation, demonstrating an ER stress response that was reversible with the ER stress inhibitor salubrinal. Assessment of pentraxin-3, an extracellular matrix protein essential for fertilization, by immunocytochemistry and Western blotting showed dramatically impaired secretion concurrent with ER stress. Mitochondrial activity in oocytes was assessed by 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide staining of inner mitochondrial membrane potential, and oocytes matured in thapsigargin or high-dose palmitic acid had significantly reduced mitochondrial activity, reduced in vitro fertilization rates, and were slower to develop to blastocysts. The deficiencies in protein secretion, mitochondrial activity, and oocyte developmental competence were each normalized by salubrinal, demonstrating that ER stress is a key mechanism mediating fatty acid-induced defects in oocyte developmental potential.
Figures
Similar articles
-
Nonesterified Fatty Acid-Induced Endoplasmic Reticulum Stress in Cattle Cumulus Oocyte Complexes Alters Cell Metabolism and Developmental Competence.Biol Reprod. 2016 Jan;94(1):23. doi: 10.1095/biolreprod.115.131862. Epub 2015 Dec 9. Biol Reprod. 2016. PMID: 26658709
-
Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model.Hum Reprod. 2019 Oct 2;34(10):1984-1998. doi: 10.1093/humrep/dez161. Hum Reprod. 2019. PMID: 31625574
-
Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors.Development. 2015 Feb 15;142(4):681-91. doi: 10.1242/dev.114850. Development. 2015. PMID: 25670793
-
The Biological Role of Hyaluronan-Rich Oocyte-Cumulus Extracellular Matrix in Female Reproduction.Int J Mol Sci. 2018 Jan 18;19(1):283. doi: 10.3390/ijms19010283. Int J Mol Sci. 2018. PMID: 29346283 Free PMC article. Review.
-
Failure to launch: aberrant cumulus gene expression during oocyte in vitro maturation.Reproduction. 2017 Mar;153(3):R109-R120. doi: 10.1530/REP-16-0426. Epub 2016 Nov 22. Reproduction. 2017. PMID: 27879344 Review.
Cited by
-
Albumin used in human IVF contain different levels of lipids and modify embryo and fetal growth in a mouse model.J Assist Reprod Genet. 2021 Sep;38(9):2371-2381. doi: 10.1007/s10815-021-02255-5. Epub 2021 Jun 11. J Assist Reprod Genet. 2021. PMID: 34114110 Free PMC article.
-
Desorption electrospray ionization mass spectrometry reveals lipid metabolism of individual oocytes and embryos.PLoS One. 2013 Sep 20;8(9):e74981. doi: 10.1371/journal.pone.0074981. eCollection 2013. PLoS One. 2013. PMID: 24073231 Free PMC article.
-
Maternal control of early embryogenesis in mammals.Reprod Fertil Dev. 2015 Jul;27(6):880-96. doi: 10.1071/RD14441. Reprod Fertil Dev. 2015. PMID: 25695370 Free PMC article. Review.
-
Role of Obesity in Female Reproduction.Int J Med Sci. 2023 Jan 31;20(3):366-375. doi: 10.7150/ijms.80189. eCollection 2023. Int J Med Sci. 2023. PMID: 36860674 Free PMC article. Review.
-
Fatty Acid Supplementation During in vitro Embryo Production Determines Cryosurvival Characteristics of Bovine Blastocysts.Front Cell Dev Biol. 2022 Mar 9;10:837405. doi: 10.3389/fcell.2022.837405. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35356284 Free PMC article.
References
-
- Cusi K. 2010. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep 10:306–315 - PubMed
-
- Waterman CL , Kian-Kai C , Griffin JL. 2010. Metabolomic strategies to study lipotoxicity in cardiovascular disease. Biochim Biophys Acta 1801:230–234 - PubMed
-
- Schaffer JE. 2003. Lipotoxicity: when tissues overeat. Curr Opin Lipidol 14:281–287 - PubMed
-
- Rich-Edwards JW , Spiegelman D , Garland M , Hertzmark E , Hunter DJ , Colditz GA , Willett WC , Wand H , Manson JE. 2002. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology 13:184–190 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

