P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice
- PMID: 22383490
- PMCID: PMC3362096
- DOI: 10.1152/ajpgi.00352.2011
P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice
Abstract
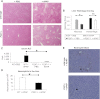
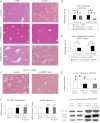
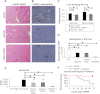
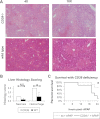
Inflammation contributes to liver injury in acetaminophen (APAP) hepatotoxicity in mice and is triggered by stimulation of immune cells. The purinergic receptor P2X7 is upstream of the nod-like receptor family, pryin domain containing-3 (NLRP3) inflammasome in immune cells and is activated by ATP and NAD that serve as damage-associated molecular patterns. APAP hepatotoxicity was assessed in mice genetically deficient in P2X7, the key inflammatory receptor for nucleotides (P2X7-/-), and in wild-type mice. P2X7-/- mice had significantly decreased APAP-induced liver necrosis. In addition, APAP-poisoned mice were treated with the specific P2X7 antagonist A438079 or etheno-NAD, a competitive antagonist of NAD. Pre- or posttreatment with A438079 significantly decreased APAP-induced necrosis and hemorrhage in APAP liver injury in wild-type but not P2X7-/- mice. Pretreatment with etheno-NAD also significantly decreased APAP-induced necrosis and hemorrhage in APAP liver injury. In addition, APAP toxicity in mice lacking the plasma membrane ecto-NTPDase CD39 (CD39-/-) that metabolizes ATP was examined in parallel with the use of soluble apyrase to deplete extracellular ATP in wild-type mice. CD39-/- mice had increased APAP-induced hemorrhage and mortality, whereas apyrase also decreased APAP-induced mortality. Kupffer cells were treated with extracellular ATP to assess P2X7-dependent inflammasome activation. P2X7 was required for ATP-stimulated IL-1β release. In conclusion, P2X7 and exposure to the ligands ATP and NAD are required for manifestations of APAP-induced hepatotoxicity.
Figures

Similar articles
-
CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury.J Hepatol. 2017 Oct;67(4):716-726. doi: 10.1016/j.jhep.2017.05.021. Epub 2017 May 26. J Hepatol. 2017. PMID: 28554875 Free PMC article.
-
Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation.Toxicol Sci. 2013 Jan;131(1):325-35. doi: 10.1093/toxsci/kfs283. Epub 2012 Sep 17. Toxicol Sci. 2013. PMID: 22986947 Free PMC article.
-
Hepatoprotective effect of allicin against acetaminophen-induced liver injury: Role of inflammasome pathway, apoptosis, and liver regeneration.J Biochem Mol Toxicol. 2020 May;34(5):e22470. doi: 10.1002/jbt.22470. Epub 2020 Feb 10. J Biochem Mol Toxicol. 2020. PMID: 32040233
-
P2X7 Receptor at the Crossroads of T Cell Fate.Int J Mol Sci. 2020 Jul 13;21(14):4937. doi: 10.3390/ijms21144937. Int J Mol Sci. 2020. PMID: 32668623 Free PMC article. Review.
-
The dual role of immune response in acetaminophen hepatotoxicity: Implication for immune pharmacological targets.Toxicol Lett. 2021 Oct 15;351:37-52. doi: 10.1016/j.toxlet.2021.08.009. Epub 2021 Aug 25. Toxicol Lett. 2021. PMID: 34454010 Review.
Cited by
-
Sterile inflammation in the liver and pancreas.J Gastroenterol Hepatol. 2013 Aug;28 Suppl 1(0 1):61-7. doi: 10.1111/jgh.12018. J Gastroenterol Hepatol. 2013. PMID: 23855298 Free PMC article. Review.
-
Lipoapoptosis induced by saturated free fatty acids stimulates monocyte migration: a novel role for Pannexin1 in liver cells.Purinergic Signal. 2015 Sep;11(3):347-59. doi: 10.1007/s11302-015-9456-5. Epub 2015 Jun 9. Purinergic Signal. 2015. PMID: 26054298 Free PMC article.
-
Application of IL-36 receptor antagonist weakens CCL20 expression and impairs recovery in the late phase of murine acetaminophen-induced liver injury.Sci Rep. 2015 Feb 17;5:8521. doi: 10.1038/srep08521. Sci Rep. 2015. PMID: 25687687 Free PMC article.
-
Purinergic signalling in liver diseases: Pathological functions and therapeutic opportunities.JHEP Rep. 2020 Jul 30;2(6):100165. doi: 10.1016/j.jhepr.2020.100165. eCollection 2020 Dec. JHEP Rep. 2020. PMID: 33103092 Free PMC article. Review.
-
Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities.Cell Mol Immunol. 2021 Jan;18(1):45-56. doi: 10.1038/s41423-020-00558-8. Epub 2020 Oct 12. Cell Mol Immunol. 2021. PMID: 33041338 Free PMC article. Review.
References
-
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol 22: 364–373, 2002 - PubMed
-
- Crispe IN. Isolation of mouse intrahepatic lymphocytes. Curr Protoc Immunol Chapter 3, Unit 3 21, 2001 [doi: 10.1002/0471142735.im0321s22] - DOI - PubMed
-
- Dear JW, Simpson KJ, Nicolai MP, Catterson JH, Street J, Huizinga T, Craig DG, Dhaliwal K, Webb S, Bateman DN, Webb DJ. Cyclophilin A is a damage-associated molecular pattern molecule that mediates acetaminophen-induced liver injury. J Immunol 187: 3347–3352, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

