p38 MAPK regulates Bax activity and apoptosis in enterocytes at baseline and after intestinal resection
- PMID: 22383494
- PMCID: PMC3362074
- DOI: 10.1152/ajpgi.00485.2011
p38 MAPK regulates Bax activity and apoptosis in enterocytes at baseline and after intestinal resection
Abstract
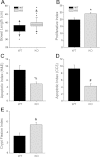
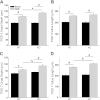
Increased apoptosis in crypt enterocytes is a key feature of intestinal adaptation following massive small bowel resection (SBR). Expression of the proapoptotic factor Bax has been shown to be required for resection-induced apoptosis. It has also been demonstrated that p38-α MAPK (p38) is necessary for Bax activation and apoptosis in vitro. The present studies were designed to test the hypothesis that p38 is a key regulator of Bax activation during adaptation after SBR in vivo. Enterocyte expression of p38 was deleted by tamoxifen administration to activate villin-Cre in adult mice with a floxed Mapk14 (p38-α) gene. Proximal 50% SBR or sham operations were performed on wild-type (WT) and p38 intestinal knockout (p38-IKO) mice under isoflurane anesthesia. Mice were killed 3 or 7 days after operation, and adaptation was analyzed by measuring intestinal morphology, proliferation, and apoptosis. Bax activity was quantified by immunoprecipitation, followed by Western blotting. After SBR, p38-IKO mice had deeper crypts, longer villi, and accelerated proliferation compared with WT controls. Rates of crypt apoptosis were significantly lower in p38-IKO mice, both at baseline and after SBR. Levels of activated Bax were twofold higher in WT mice after SBR relative to sham. In contrast, activated Bax levels were reduced by 67% in mice after p38 MAPK deletion. Deleted p38 expression within the intestinal epithelium leads to enhanced adaptation and reduced levels of enterocyte apoptosis after massive intestinal resection. p38-regulated Bax activation appears to be an important mechanism underlying resection-induced apoptosis.
Figures




Similar articles
-
Preventing enterocyte apoptosis after massive small bowel resection does not enhance adaptation of the intestinal mucosa.J Pediatr Surg. 2004 Jun;39(6):907-11; discussion 907-11. doi: 10.1016/j.jpedsurg.2004.02.007. J Pediatr Surg. 2004. PMID: 15185223
-
Influence of the site of small bowel resection on intestinal epithelial cell apoptosis.Pediatr Surg Int. 2006 Jan;22(1):37-42. doi: 10.1007/s00383-005-1576-5. Pediatr Surg Int. 2006. PMID: 16307277 Free PMC article.
-
Bax is required for increased enterocyte apoptosis after massive small bowel resection.Surgery. 2000 Aug;128(2):165-70. doi: 10.1067/msy.2000.107370. Surgery. 2000. PMID: 10922987
-
Effect of massive small bowel resection on the Bax/Bcl-w ratio and enterocyte apoptosis.J Gastrointest Surg. 2000 Jan-Feb;4(1):93-100. doi: 10.1016/s1091-255x(00)80038-4. J Gastrointest Surg. 2000. PMID: 10631368
-
Epidermal growth factor alters the bax:bcl-w ratio following massive small bowel resection.J Surg Res. 2000 Jun 1;91(1):38-42. doi: 10.1006/jsre.2000.5897. J Surg Res. 2000. PMID: 10816347
Cited by
-
SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis.Redox Biol. 2020 Oct;37:101760. doi: 10.1016/j.redox.2020.101760. Epub 2020 Oct 15. Redox Biol. 2020. PMID: 33096425 Free PMC article.
-
The Effect of Caffeic Acid on Spermatogonial Stem Cell-type A Cryopreservation.Rep Biochem Mol Biol. 2018 Oct;7(1):85-93. Rep Biochem Mol Biol. 2018. PMID: 30324122 Free PMC article.
-
Insulin-like growth factor 2 and its enterocyte receptor are not required for adaptation in response to massive small bowel resection.J Pediatr Surg. 2014 Jun;49(6):966-70; discussion 970. doi: 10.1016/j.jpedsurg.2014.01.035. Epub 2014 Jan 31. J Pediatr Surg. 2014. PMID: 24888844 Free PMC article.
-
Effects of high-fat diet on liver injury after small bowel resection.J Pediatr Surg. 2020 Jun;55(6):1099-1106. doi: 10.1016/j.jpedsurg.2020.02.037. Epub 2020 Feb 28. J Pediatr Surg. 2020. PMID: 32164985 Free PMC article.
-
Both epidermal growth factor and insulin-like growth factor receptors are dispensable for structural intestinal adaptation.J Pediatr Surg. 2015 Jun;50(6):943-7. doi: 10.1016/j.jpedsurg.2015.03.015. Epub 2015 Mar 14. J Pediatr Surg. 2015. PMID: 25818318 Free PMC article.
References
-
- Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem 276: 11615–11623, 2001 - PubMed
-
- Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther 279: 1453–1461, 1996 - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007 - PubMed
-
- Bernal NP, Stehr W, Coyle R, Erwin CR, Warner BW. Epidermal growth factor receptor signaling regulates Bax and Bcl-w expression and apoptotic responses during intestinal adaptation in mice. Gastroenterology 130: 412–423, 2006 - PubMed
-
- Bernal NP, Stehr W, Profitt S, Erwin CR, Warner BW. Combined pharmacotherapy that increases proliferation and decreases apoptosis optimally enhances intestinal adaptation. J Pediatr Surg 41: 719–724, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

