Unloading stress disturbs muscle regeneration through perturbed recruitment and function of macrophages
- PMID: 22383511
- PMCID: PMC3785183
- DOI: 10.1152/japplphysiol.00103.2012
Unloading stress disturbs muscle regeneration through perturbed recruitment and function of macrophages
Abstract
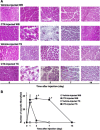
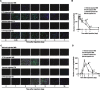
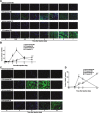
Skeletal muscle is one of the most sensitive tissues to mechanical loading, and unloading inhibits the regeneration potential of skeletal muscle after injury. This study was designed to elucidate the specific effects of unloading stress on the function of immunocytes during muscle regeneration after injury. We examined immunocyte infiltration and muscle regeneration in cardiotoxin (CTX)-injected soleus muscles of tail-suspended (TS) mice. In CTX-injected TS mice, the cross-sectional area of regenerating myofibers was smaller than that of weight-bearing (WB) mice, indicating that unloading delays muscle regeneration following CTX-induced skeletal muscle damage. Delayed infiltration of macrophages into the injured skeletal muscle was observed in CTX-injected TS mice. Neutrophils and macrophages in CTX-injected TS muscle were presented over a longer period at the injury sites compared with those in CTX-injected WB muscle. Disturbance of activation and differentiation of satellite cells was also observed in CTX-injected TS mice. Further analysis showed that the macrophages in soleus muscles were mainly Ly-6C-positive proinflammatory macrophages, with high expression of tumor necrosis factor-α and interleukin-1β, indicating that unloading causes preferential accumulation and persistence of proinflammatory macrophages in the injured muscle. The phagocytic and myotube formation properties of macrophages from CTX-injected TS skeletal muscle were suppressed compared with those from CTX-injected WB skeletal muscle. We concluded that the disturbed muscle regeneration under unloading is due to impaired macrophage function, inhibition of satellite cell activation, and their cooperation.
Figures




References
-
- Authier FJ, Chazaud B, Plonquet A, Eliezer-Vanerot MC, Poron F, Belec L, Barlovatz-Meimon G, Gherardi RK. Differential expression of the IL-1 system components during in vitro myogenesis: implication of IL-1β in induction of myogenic cell apoptosis. Cell Death Differ 6: 1012–1021, 1999 - PubMed
-
- Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res 95: 858–866, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

