Twelve weeks of pioglitazone therapy significantly attenuates dysmetabolism and reduces inflammation in continuous ambulatory peritoneal dialysis patients--a randomized crossover trial
- PMID: 22383630
- PMCID: PMC3524878
- DOI: 10.3747/pdi.2011.00116
Twelve weeks of pioglitazone therapy significantly attenuates dysmetabolism and reduces inflammation in continuous ambulatory peritoneal dialysis patients--a randomized crossover trial
Abstract
Background: The aim of the present study was to investigate the effect of oral pioglitazone (PIO) on lipid metabolism, insulin resistance, inflammation, and adipokine metabolism in continuous ambulatory peritoneal dialysis (CAPD) patients.
Methods: In this randomized crossover trial, 36 CAPD patients with serum triglyceride levels above 1.8 mmol/L were randomly assigned to receive either oral PIO 15 mg once daily or no PIO for 12 weeks. Then, after a 4-week washout, the patients were switched to the alternative regimen. The primary endpoint was change in serum triglycerides during the PIO regimen compared with no PIO. Secondary endpoints included changes in other lipid levels, homeostatic model assessment of insulin resistance (HOMA-IR), adipocytokines, and C-reactive protein (CRP).
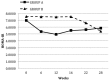
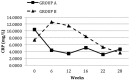
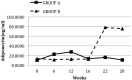
Results: All 36 CAPD patients (age: 64 ± 11 years; 33% men; 27.8% with diabetes mellitus) completed the study. Comparing patients after PIO and no PIO therapy, we found no significant differences in mean serum triglycerides (3.83 ± 1.49 mmol/L vs 3.51 ± 1.98 mmol/L, p = 0.2). However, mean high-density lipoprotein (0.94 ± 0.22 mmol/L vs 1.00 ± 0.21 mmol/L, p = 0.004) and median total adiponectin [10.34 μg/mL (range: 2.59 - 34.48 μg/mL) vs 30.44 μg/mL (3.47 - 93.41 μg/mL), p < 0.001] increased significantly. Median HOMA-IR [7.51 (1.39 - 45.23) vs 5.38 (0.97 - 14.95), p = 0.006], mean fasting blood glucose (7.31 ± 2.57 mmol/L vs 6.60 ± 2.45 mmol/L, p = 0.01), median CRP [8.78 mg/L (0.18 - 53 mg/L) vs 3.50 mg/L (0.17 - 26.30 mg/L), p = 0.005], and mean resistin (32.70 ± 17.17 ng/mL vs 28.79 ± 11.83 ng/mL, p = 0.02) all declined. The PIO was well tolerated, with only one adverse event: lower-extremity edema in a patient with low residual renal function.
Conclusions: Blood triglycerides were not altered after 12 weeks of PIO 15 mg once daily in CAPD patients, but parameters of dysmetabolism were markedly improved, including insulin resistance, inflammation, and adipokine balance, suggesting that PIO could be of value for this high-risk patient group. Larger, more definitive studies are needed to confirm these findings.
Figures
References
-
- Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl 2006; (103):S3–11 - PubMed
-
- Huang CC, Cheng KF, Wu HD. Survival analysis: comparing peritoneal dialysis and hemodialysis in Taiwan. Perit Dial Int 2008; 28(Suppl 3):S15–20 - PubMed
-
- Fortes PC, de Moraes TP, Mendes JG, Stinghen AE, Ribeiro SC, Pecoits-Filho R. Insulin resistance and glucose homeostasis in peritoneal dialysis. Perit Dial Int 2009; 29(Suppl 2):S145–8 - PubMed
-
- Shen Y, Peake PW, Kelly JJ. Should we quantify insulin resistance in patients with renal disease? Nephrology (Carlton) 2005; 10:599–605 - PubMed
-
- Pecoits-Filho R, Stenvinkel P, Wang AY, Heimbürger O, Lindholm B. Chronic inflammation in peritoneal dialysis: the search for the holy grail? Perit Dial Int 2004; 24:327–39 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

