ADRB2 polymorphisms and budesonide/formoterol responses in COPD
- PMID: 22383665
- PMCID: PMC3425335
- DOI: 10.1378/chest.11-1655
ADRB2 polymorphisms and budesonide/formoterol responses in COPD
Abstract
Background: Effects of β(2)-adrenergic receptor gene (ADRB2) polymorphism on therapeutic responses to long-acting β(2)-adrenergic agonists have not been evaluated in long-term COPD trials. We aimed to investigate the effects of the ADRB2 Gly16Arg polymorphism on response to formoterol alone or in combination with the inhaled corticosteroid budesonide in patients with COPD.
Methods: Patients ≥ 40 years of age with moderate to very severe COPD from the 12-month trial I (NCT00206167) or the 6-month trial II (NCT00206154) were randomly assigned to bid budesonide/formoterol pressurized metered-dose inhaler (pMDI) 320/9 μg or 160/9 μg, budesonide pMDI 320 μg + formoterol dry powder inhaler 9 μg (trial II), budesonide pMDI 320 μg (trial II), formoterol dry powder inhaler 9 μg, or placebo. The effect of Gly16Arg on predose FEV(1) and 1-h postdose FEV(1), exacerbations, diary variables, and adverse events were analyzed.
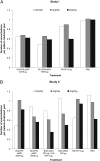
Results: No significant interaction between genotype and treatment response was observed for predose (P ≥ .197) or postdose FEV(1) (P ≥ .125) in either pharmacogenetic study (n = 2,866). The number of COPD exacerbations per patient-treatment year was low and similar across genotypes for the active treatment groups (both studies). Percentages of patients with adverse events were similar across Gly16Arg genotype groups for each treatment.
Conclusion: Therapeutic response and tolerability to long-term treatment with formoterol alone or in combination with budesonide was not modified by ADRB2 Gly16Arg genotype in two large independent pharmacogenetic studies in patients with moderate to very severe COPD.
Figures


References
-
- Matheson MC, Ellis JA, Raven J, Johns DP, Walters EH, Abramson MJ. Beta2-adrenergic receptor polymorphisms are associated with asthma and COPD in adults. J Hum Genet. 2006;51(11):943-951 - PubMed
-
- Israel E, Drazen JM, Liggett SB, et al. ; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162(1):75-80 - PubMed
-
- Israel E, Chinchilli VM, Ford JG, et al. ; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364(9444):1505-1512 - PubMed

