Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation
- PMID: 22383668
- PMCID: PMC3435140
- DOI: 10.1378/chest.11-2672
Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation
Abstract
Background: Ivacaftor (VX-770) is a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator that was approved in the United States for the treatment of cystic fibrosis (CF) in patients ≥ 6 years of age who have a G551D mutation; however, the most prevalent disease-causing CFTR mutation, F508del, causes a different functional defect. The objectives of this study were to evaluate the safety of ivacaftor in a larger population and for a longer time period than tested previously and to assess the efficacy of ivacaftor in subjects with CF who are homozygous for F508del-CFTR.
Methods: This was a phase 2 study with a 16-week randomized (4:1), double-blind, placebo-controlled period (part A) and an open-label extension (part B) for subjects who met prespecified criteria.
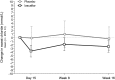
Results: Part A: The safety profile of ivacaftor was comparable to that of the placebo. The overall adverse event frequency was similar in the ivacaftor (87.5%) and placebo (89.3%) groups through 16 weeks. The difference in the change of FEV₁ % predicted from baseline through week 16 (primary end point) between the ivacaftor and placebo groups was 1.7% (P = .15). Sweat chloride, a biomarker of CFTR activity, showed a small reduction in the ivacaftor vs placebo groups of -2.9 mmol/L (P = .04) from baseline through week 16. Part B: No new safety signals were identified. The changes in FEV₁ or sweat chloride in part A were not sustained with ivacaftor treatment from week 16 to week 40.
Conclusions: These results expand the safety information for ivacaftor and support its continued evaluation. Lack of a clinical effect suggests that a CFTR potentiator alone is not an effective therapeutic approach for patients who have CF and are homozygous for F508del-CFTR.
Trial registry: ClinicalTrials.gov; No.: NCT00953706; URL: www.clinicaltrials.gov.
Figures
References
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992-2001 - PubMed
-
- McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361(9370):1671-1676 - PubMed
-
- Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245(4922):1073-1080 - PubMed
-
- Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066-1073 - PubMed
-
- Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245(4922):1059-1065 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

