Regulation of skeletal muscle lipolysis and oxidative metabolism by the co-lipase CGI-58
- PMID: 22383684
- PMCID: PMC3329383
- DOI: 10.1194/jlr.M019182
Regulation of skeletal muscle lipolysis and oxidative metabolism by the co-lipase CGI-58
Abstract
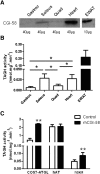
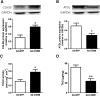
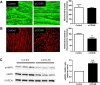
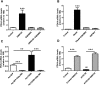
We investigated here the specific role of CGI-58 in the regulation of energy metabolism in skeletal muscle. We first examined CGI-58 protein expression in various muscle types in mice, and next modulated CGI-58 expression during overexpression and knockdown studies in human primary myotubes and evaluated the consequences on oxidative metabolism. We observed a preferential expression of CGI-58 in oxidative muscles in mice consistent with triacylglycerol hydrolase activity. We next showed by pulse-chase that CGI-58 overexpression increased by more than 2-fold the rate of triacylglycerol (TAG) hydrolysis, as well as TAG-derived fatty acid (FA) release and oxidation. Oppositely, CGI-58 silencing reduced TAG hydrolysis and TAG-derived FA release and oxidation (-77%, P < 0.001), whereas it increased glucose oxidation and glycogen synthesis. Interestingly, modulations of CGI-58 expression and FA release are reflected by changes in pyruvate dehydrogenase kinase 4 gene expression. This regulation involves the activation of the peroxisome proliferator activating receptor-δ (PPARδ) by lipolysis products. Altogether, these data reveal that CGI-58 plays a limiting role in the control of oxidative metabolism by modulating FA availability and the expression of PPARδ-target genes, and highlight an important metabolic function of CGI-58 in skeletal muscle.
Figures



References
-
- Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737 - PubMed
-
- Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386 - PubMed
-
- Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3: 309–319 - PubMed
-
- Lefevre C., Jobard F., Caux F., Bouadjar B., Karaduman A., Heilig R., Lakhdar H., Wollenberg A., Verret J. L., Weissenbach J., et al. 2001. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am. J. Hum. Genet. 69: 1002–1012 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

