ApoE promotes hepatic selective uptake but not RCT due to increased ABCA1-mediated cholesterol efflux to plasma
- PMID: 22383685
- PMCID: PMC3329392
- DOI: 10.1194/jlr.M020743
ApoE promotes hepatic selective uptake but not RCT due to increased ABCA1-mediated cholesterol efflux to plasma
Abstract
ApoE plays an important role in lipoprotein metabolism. This study investigated the effects of adenovirus-mediated human apoE overexpression (AdhApoE3) on sterol metabolism and in vivo reverse cholesterol transport (RCT). In wild-type mice, AdhApoE3 resulted in decreased HDL cholesterol levels and a shift toward larger HDL in plasma, whereas hepatic cholesterol content increased (P < 0.05). These effects were dependent on scavenger receptor class B type I (SR-BI) as confirmed using SR-BI-deficient mice. Kinetic studies demonstrated increased plasma HDL cholesteryl ester catabolic rates (P < 0.05) and higher hepatic selective uptake of HDL cholesteryl esters in AdhApoE3-injected wild-type mice (P < 0.01). However, biliary and fecal sterol output as well as in vivo macrophage-to-feces RCT studied with (3)H-cholesterol-loaded mouse macrophage foam cells remained unchanged upon human apoE overexpression. Similar results were obtained using hApoE3 overexpression in human CETP transgenic mice. However, blocking ABCA1-mediated cholesterol efflux from hepatocytes in AdhApoE3-injected mice using probucol increased biliary cholesterol secretion (P < 0.05), fecal neutral sterol excretion (P < 0.05), and in vivo RCT (P < 0.01), specifically within neutral sterols. These combined data demonstrate that systemic apoE overexpression increases i) SR-BI-mediated selective uptake into the liver and ii) ABCA1-mediated efflux of RCT-relevant cholesterol from hepatocytes back to the plasma compartment, thereby resulting in unchanged fecal mass sterol excretion and overall in vivo RCT.
Figures

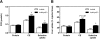




References
-
- Mahley R. W. 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 240: 622–630 - PubMed
-
- Newman T. C., Dawson P. A., Rudel L. L., Williams D. L. 1985. Quantitation of apolipoprotein E mRNA in the liver and peripheral tissues of nonhuman primates. J. Biol. Chem. 260: 2452–2457 - PubMed
-
- Basu S. K., Goldstein J. L., Brown M. S. 1983. Independent pathways for secretion of cholesterol and apolipoprotein E by macrophages. Science. 219: 871–873 - PubMed
-
- Plump A. S., Smith J. D., Hayek T., Aalto-Setala K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. 1992. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 71: 343–353 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

