A novel mRNA binding protein complex promotes localized plasminogen activator inhibitor-1 accumulation at the myoendothelial junction
- PMID: 22383705
- PMCID: PMC3331922
- DOI: 10.1161/ATVBAHA.112.246371
A novel mRNA binding protein complex promotes localized plasminogen activator inhibitor-1 accumulation at the myoendothelial junction
Abstract
Objective: Plasminogen activator inhibitor-1 (PAI-1) has previously been shown to be key to the formation of myoendothelial junctions (MEJs) in normal and pathological states (eg, obesity). We therefore sought to identify the mechanism whereby PAI-1 could be selectively accumulated at the MEJ.
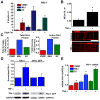
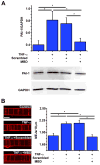
Methods and results: We identified PAI-1 protein enrichment at the MEJ in obese mice and in response to tumor necrosis factor (TNF-α) with a vascular cell coculture. However, PAI-1 mRNA was also found at the MEJ and transfection with a PAI-1-GFP with TNF-α did not demonstrate trafficking of the protein to the MEJ. We therefore hypothesized the PAI-1 mRNA was being locally translated and identified serpine binding protein-1, which stabilizes PAI-1 mRNA, as being enriched in obese mice and after treatment with TNF-α, whereas Staufen, which degrades PAI-1 mRNA, was absent in obese mice and after TNF-α application. We identified nicotinamide phosphoribosyl transferase as a serpine binding protein-1 binding partner with a functional τ-like microtubule binding domain. Application of peptides against the microtubule binding domain significantly decreased the number of MEJs and the amount of PAI-1 at the MEJ.
Conclusions: We conclude that PAI-1 can be locally translated at the MEJ as a result of a unique mRNA binding protein complex.
Figures




Similar articles
-
Plasminogen activator inhibitor-1 regulates myoendothelial junction formation.Circ Res. 2010 Apr 2;106(6):1092-102. doi: 10.1161/CIRCRESAHA.109.215723. Epub 2010 Feb 4. Circ Res. 2010. PMID: 20133900 Free PMC article.
-
Recombinant PAI-1 therapy restores myoendothelial junctions and erectile function in PAI-1-deficient mice.Andrologia. 2015 Dec;47(10):1147-52. doi: 10.1111/and.12395. Epub 2014 Dec 29. Andrologia. 2015. PMID: 25557984 Free PMC article.
-
Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo. Induction by tumor necrosis factor-alpha and lipopolysaccharide.J Clin Invest. 1996 Jan 1;97(1):37-46. doi: 10.1172/JCI118404. J Clin Invest. 1996. PMID: 8550848 Free PMC article.
-
The 'PAI-1 paradox' in vascular remodeling.Thromb Haemost. 2008 Dec;100(6):984-91. Thromb Haemost. 2008. PMID: 19132221 Review.
-
Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells.Thromb Haemost. 2008 Dec;100(6):976-83. Thromb Haemost. 2008. PMID: 19132220 Free PMC article. Review.
Cited by
-
Myoendothelial Junctions of Mature Coronary Vessels Express Notch Signaling Proteins.Front Physiol. 2020 Feb 4;11:29. doi: 10.3389/fphys.2020.00029. eCollection 2020. Front Physiol. 2020. PMID: 32116749 Free PMC article.
-
Role of plasminogen activator inhibitor-1 in senescence and aging.Semin Thromb Hemost. 2014 Sep;40(6):645-51. doi: 10.1055/s-0034-1387883. Epub 2014 Aug 31. Semin Thromb Hemost. 2014. PMID: 25173500 Free PMC article. Review.
-
Heterocellular Contact Can Dictate Arterial Function.Circ Res. 2019 May 10;124(10):1473-1481. doi: 10.1161/CIRCRESAHA.118.313926. Circ Res. 2019. PMID: 30900949 Free PMC article.
-
Endothelium-Derived Hyperpolarization and Coronary Vasodilation: Diverse and Integrated Roles of Epoxyeicosatrienoic Acids, Hydrogen Peroxide, and Gap Junctions.Microcirculation. 2016 Jan;23(1):15-32. doi: 10.1111/micc.12255. Microcirculation. 2016. PMID: 26541094 Free PMC article. Review.
-
The myoendothelial junction: connections that deliver the message.Physiology (Bethesda). 2014 Jul;29(4):242-9. doi: 10.1152/physiol.00042.2013. Physiology (Bethesda). 2014. PMID: 24985328 Free PMC article. Review.
References
-
- Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. - PubMed
-
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. - PubMed
-
- Taugner R, Kirchheim H, Forssmann WG. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and tupaia belangeri. Cell Tissue Res. 1984;235:319–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

