Differential effect of intrauterine hypoxia on caspase 3 and DNA fragmentation in fetal guinea pig hearts and brains
- PMID: 22383778
- PMCID: PMC3343149
- DOI: 10.1177/1933719111420883
Differential effect of intrauterine hypoxia on caspase 3 and DNA fragmentation in fetal guinea pig hearts and brains
Abstract
The aim of this study is to quantify the effect of intrauterine hypoxia (HPX) and the role of nitric oxide (NO) on the apoptotic enzyme, caspase 3, and DNA fragmentation in fetal heart and brain. Hypoxia and NO are important regulators of apoptosis, although this has been little studied in the fetal organs. We investigated the effect of intrauterine HPX on apoptosis and the role of NO in both fetal hearts and brains. Pregnant guinea pigs were exposed to room temperature (N = 14) or 10.5% O₂ (N = 12) for 14 days prior to term (term = 65 days) and administered water or L-N6-(1-iminoethyl)-lysine (LNIL), an inducible nitric oxide synthase (iNOS) inhibitor, for 10 days. Fetal hearts and brains were excised from anesthetized near-term fetuses for study. Chronic HPX decreased pro- and active caspase 3, caspase 3 activity, and DNA fragmentation levels in fetal hearts compared with normoxic controls. L-N6-(1-iminoethyl)-lysine prevented the HPX-induced decrease in caspase 3 activity but did not alter DNA fragmentation levels. In contrast, chronic HPX increased both apoptotic indices in fetal brains, which were inhibited by LNIL. Thus, the effect of HPX on apoptosis differs between fetal organs, and NO may play an important role in modulating these effects.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


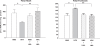

Similar articles
-
Chronic hypoxia increases inducible NOS-derived nitric oxide in fetal guinea pig hearts.Pediatr Res. 2009 Feb;65(2):188-92. doi: 10.1203/PDR.0b013e31818d6ad0. Pediatr Res. 2009. PMID: 19047955 Free PMC article.
-
Chronic hypoxia decreases endothelial nitric oxide synthase protein expression in fetal guinea pig hearts.J Soc Gynecol Investig. 2005 Sep;12(6):388-95. doi: 10.1016/j.jsgi.2005.04.011. J Soc Gynecol Investig. 2005. PMID: 15982907
-
Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts.Pediatr Res. 2012 Jan;71(1):25-31. doi: 10.1038/pr.2011.10. Pediatr Res. 2012. PMID: 22289847
-
Hypoxia and fetal heart development.Curr Mol Med. 2010 Oct;10(7):653-66. doi: 10.2174/156652410792630643. Curr Mol Med. 2010. PMID: 20712587 Free PMC article. Review.
-
Impact of Chronic Fetal Hypoxia and Inflammation on Cardiac Pacemaker Cell Development.Cells. 2020 Mar 17;9(3):733. doi: 10.3390/cells9030733. Cells. 2020. PMID: 32192015 Free PMC article. Review.
Cited by
-
Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic.J Physiol. 2018 Dec;596(23):5535-5569. doi: 10.1113/JP274948. Epub 2018 May 30. J Physiol. 2018. PMID: 29633280 Free PMC article.
-
Spermidine Prevents Heart Injury in Neonatal Rats Exposed to Intrauterine Hypoxia by Inhibiting Oxidative Stress and Mitochondrial Fragmentation.Oxid Med Cell Longev. 2019 May 14;2019:5406468. doi: 10.1155/2019/5406468. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31217839 Free PMC article.
-
Near to One's Heart: The Intimate Relationship Between the Placenta and Fetal Heart.Front Physiol. 2018 Jun 26;9:629. doi: 10.3389/fphys.2018.00629. eCollection 2018. Front Physiol. 2018. PMID: 29997513 Free PMC article. Review.
-
Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection.Mol Neurobiol. 2014 Feb;49(1):222-33. doi: 10.1007/s12035-013-8514-7. Epub 2013 Jul 28. Mol Neurobiol. 2014. PMID: 23893294
References
-
- Jones RD, Morice AH, Emery CJ. Effects of perinatal exposure to hypoxia upon the pulmonary circulation of the adult rat. Physiol Res. 2004;53(1):11–17 - PubMed
-
- VanWijk MJ, Kublickiene K, Boer K, VanBavel E. Vascular function in preeclampsia. Cardiovasc Res. 2000;47(1):38–48 - PubMed
-
- Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A Mol Integr Physiol. 1998;119(3):717–723 - PubMed
-
- Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig. 2005;12(1):2–13 - PubMed
-
- Louey S, Thornburg KL. The prenatal environment and later cardiovascular disease. Early Hum Dev. 2005;81(9):745–751 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

