Impact of experimental diabetes on the maternal uterine vascular remodeling during rat pregnancy
- PMID: 22383782
- PMCID: PMC3343150
- DOI: 10.1177/1933719111424435
Impact of experimental diabetes on the maternal uterine vascular remodeling during rat pregnancy
Abstract
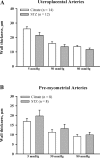
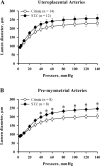
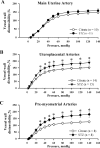
Normal pregnancy is associated with an increase in uteroplacental blood flow in part due to growth and remodeling of the maternal uterine vasculature. In this study, we characterized the effect of diabetic pregnancy on vascular growth of the maternal uterine vasculature and on the passive mechanical properties of the uterine resistance arteries. Diabetes was induced in pregnant rats by injection of streptozotocin and confirmed by development of hyperglycemia. Fetuses of diabetic rats were significantly smaller and placentas larger compared to controls. Pregnancy-induced axial elongation of the mesometrial uterine vasculature was not altered by diabetes. Vascular wall thickness was unchanged between groups. Wall distensibility was increased and the rate constant of an exponential function fitted to stress-strain curve was significantly reduced demonstrating decreased wall stiffness in diabetic uterine radial arteries compared to controls. We conclude that experimental diabetes in rat pregnancy does not compromise the growth of maternal uterine vasculature but alters passive mechanical properties of the uterine radial arteries.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
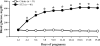



Similar articles
-
Role of impaired endothelial cell Ca(2+) signaling in uteroplacental vascular dysfunction during diabetic rat pregnancy.Am J Physiol Heart Circ Physiol. 2013 Apr 1;304(7):H935-45. doi: 10.1152/ajpheart.00513.2012. Epub 2013 Feb 1. Am J Physiol Heart Circ Physiol. 2013. PMID: 23376827 Free PMC article.
-
Decreased uterine blood flow in the diabetic pregnant rat does not modify the augmented glucose transfer to the fetus.Biol Neonate. 1985;48(4):197-203. doi: 10.1159/000242172. Biol Neonate. 1985. PMID: 4063414
-
Uterine distension differentially affects remodelling and distensibility of the uterine vasculature in non-pregnant rats.Reprod Fertil Dev. 2012;24(6):835-42. doi: 10.1071/RD11208. Reprod Fertil Dev. 2012. PMID: 22781934
-
Diabetes and the maternal resistance vasculature.Clin Sci (Lond). 2001 Dec;101(6):719-29. Clin Sci (Lond). 2001. PMID: 11724662 Review.
-
Physiological remodelling of the maternal uterine circulation during pregnancy.Basic Clin Pharmacol Toxicol. 2012 Jan;110(1):12-8. doi: 10.1111/j.1742-7843.2011.00793.x. Epub 2011 Oct 21. Basic Clin Pharmacol Toxicol. 2012. PMID: 21902814 Review.
Cited by
-
Modeling Trophoblast Cell-Guided Uterine Spiral Artery Transformation in the Rat.Int J Mol Sci. 2022 Mar 9;23(6):2947. doi: 10.3390/ijms23062947. Int J Mol Sci. 2022. PMID: 35328368 Free PMC article. Review.
-
Augmented dilation to nitric oxide in uterine arteries from rats with type 2 diabetes: implications for vascular adaptations to pregnancy.Am J Physiol Heart Circ Physiol. 2014 Feb 15;306(4):H610-8. doi: 10.1152/ajpheart.00588.2013. Epub 2013 Dec 13. Am J Physiol Heart Circ Physiol. 2014. PMID: 24337459 Free PMC article.
-
Impact of Immune Deficiency on Remodeling of Maternal Resistance Vasculature 4 Weeks Postpartum in Mice.Reprod Sci. 2017 Apr;24(4):514-525. doi: 10.1177/1933719116678691. Epub 2017 Jan 19. Reprod Sci. 2017. PMID: 27899739 Free PMC article.
-
Immune Mechanisms Linking Obesity and Preeclampsia.Biomolecules. 2015 Nov 12;5(4):3142-76. doi: 10.3390/biom5043142. Biomolecules. 2015. PMID: 26569331 Free PMC article. Review.
-
Deletion of RSK2 kinase alleviates age-dependent hypertension.bioRxiv [Preprint]. 2025 Mar 14:2025.03.12.642932. doi: 10.1101/2025.03.12.642932. bioRxiv. 2025. PMID: 40161735 Free PMC article. Preprint.
References
-
- Assali NS, Douglass RA, Jr, Baird WW, Nicholson DB, Suyemoto R. Measurement of uterine blood flow and uterine metabolism. IV. Results in normal pregnancy. Am J Obstet Gynecol. 1953; 66(2): 248–253 - PubMed
-
- Assali NS, Rauramo L, Peltonen T. Measurement of uterine blood flow and uterine metabolism. VIII. Uterine and fetal blood flow and oxygen consumption in early human pregnancy. Am J Obstet Gynecol. 1960; 79: 86–98 - PubMed
-
- Ramsey E. Placental vasculature and circulation. In: Handbook of Physiology. Endocrinology Female Reproductive System. Bethesda, MD: Am Physiol Soc. 1973; vol. II, pt 2, 323–337
-
- Meschia G. Circulation to female reproductive organs. In: Handbook of Physiology. The Cardiovascular System III. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am Physiol Soc. 1983; sect. 2, vol. III, pt. 1, 241–269
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

