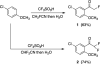Fluoro-substituted ketones from nitriles using acidic and basic reaction conditions
- PMID: 22383858
- PMCID: PMC3286112
- DOI: 10.1016/j.tetlet.2011.07.125
Fluoro-substituted ketones from nitriles using acidic and basic reaction conditions
Abstract
Fluoro-substituted aliphatic nitriles are shown to undergo the Houben-Hoesch reactions with arenes in CF(3)SO(3)H to give fluoro-substituted ketones in good yields. The fluorine substituents appear to enhance the reactivities of the nitriles (and the nitrilium ion intermediates) compared to similar aliphatic nitriles. Fluoro-substituted ketones are also shown to be accessible through the reactions of organometallic reagents and fluoro-substituted nitriles.
Figures
References
-
- Hoesch K. Ber. Dtsch. Chem. Ges. 1915;48:1122.
- Houben J. Ber. Dtsch. Chem. Ges. 1926;59:2878.
- Smith MB, March J. March's Advanced Organic Chemistry. 6th Ed. Wiley; NY: 2007. pp. 732–733.
- Mullins RJ, O'Reilly MC. In: Name Reactions for Carbocyclic Ring Formations. Li JJ, editor. Wiley; NY: 2010. pp. 675–687.
- Ruske W. In: Friedel-Crafts and Related Reactions. Olah GA, editor. Volume III. Interscience; New York: 1964. pp. 383–497.
-
- Booth BL, Noori GFM. J. Chem. Soc. Perkin Trans I. 1980:2894.
- Sanchez-Viesca F, Gomez MR, Berros M. Org. Prep. Proc. Int. 2004;36:135.
-
- Neill AB, Amstutz ED. J. Am. Chem. Soc. 1951;73:3687.
- Kobayashi Y, Nakatani T, Tanaka R, Okada M, Torii E, Harayama T, Kimachi T. Tetrahedron. 2011;67:3457.
-
- Sato Y, Yato M, Ohwada T, Saito S, Shudo K. J. Am. Chem. Soc. 1995;117:3037.
Grants and funding
LinkOut - more resources
Full Text Sources