The invasive capacity of HPV transformed cells requires the hDlg-dependent enhancement of SGEF/RhoG activity
- PMID: 22383878
- PMCID: PMC3285591
- DOI: 10.1371/journal.ppat.1002543
The invasive capacity of HPV transformed cells requires the hDlg-dependent enhancement of SGEF/RhoG activity
Abstract
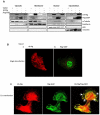
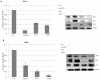
A major target of the HPV E6 oncoprotein is the human Discs Large (hDlg) tumour suppressor, although how this interaction contributes to HPV-induced malignancy is still unclear. Using a proteomic approach we show that a strong interacting partner of hDlg is the RhoG-specific guanine nucleotide exchange factor SGEF. The interaction between hDlg1 and SGEF involves both PDZ and SH3 domain recognition, and directly contributes to the regulation of SGEF's cellular localization and activity. Consistent with this, hDlg is a strong enhancer of RhoG activity, which occurs in an SGEF-dependent manner. We also show that HPV-18 E6 can interact indirectly with SGEF in a manner that is dependent upon the presence of hDlg and PDZ binding capacity. In HPV transformed cells, E6 maintains a high level of RhoG activity, and this is dependent upon the presence of hDlg and SGEF, which are found in complex with E6. Furthermore, we show that E6, hDlg and SGEF each directly contributes to the invasive capacity of HPV-16 and HPV-18 transformed tumour cells. These studies demonstrate that hDlg has a distinct oncogenic function in the context of HPV induced malignancy, one of the outcomes of which is increased RhoG activity and increased invasive capacity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


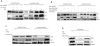




Similar articles
-
Structural insights into a wildtype domain of the oncoprotein E6 and its interaction with a PDZ domain.PLoS One. 2013 Apr 30;8(4):e62584. doi: 10.1371/journal.pone.0062584. Print 2013. PLoS One. 2013. PMID: 23638119 Free PMC article.
-
The Src homology 3 domain-containing guanine nucleotide exchange factor is overexpressed in high-grade gliomas and promotes tumor necrosis factor-like weak inducer of apoptosis-fibroblast growth factor-inducible 14-induced cell migration and invasion via tumor necrosis factor receptor-associated factor 2.J Biol Chem. 2013 Jul 26;288(30):21887-97. doi: 10.1074/jbc.M113.468686. Epub 2013 Jun 17. J Biol Chem. 2013. PMID: 23775076 Free PMC article.
-
Association of cottontail rabbit papillomavirus E6 oncoproteins with the hDlg/SAP97 tumor suppressor.J Cell Biochem. 2005 Apr 1;94(5):1038-45. doi: 10.1002/jcb.20383. J Cell Biochem. 2005. PMID: 15669058
-
Tyrosine Phosphorylation of SGEF Regulates RhoG Activity and Cell Migration.PLoS One. 2016 Jul 20;11(7):e0159617. doi: 10.1371/journal.pone.0159617. eCollection 2016. PLoS One. 2016. PMID: 27437949 Free PMC article.
-
Proteasome-mediated regulation of the hDlg tumour suppressor protein.J Cell Sci. 2001 Dec;114(Pt 23):4285-92. doi: 10.1242/jcs.114.23.4285. J Cell Sci. 2001. PMID: 11739660
Cited by
-
Structural insights into a wildtype domain of the oncoprotein E6 and its interaction with a PDZ domain.PLoS One. 2013 Apr 30;8(4):e62584. doi: 10.1371/journal.pone.0062584. Print 2013. PLoS One. 2013. PMID: 23638119 Free PMC article.
-
Regulation of circular dorsal ruffles, macropinocytosis, and cell migration by RhoG and its exchange factor, Trio.Mol Biol Cell. 2017 Jul 1;28(13):1768-1781. doi: 10.1091/mbc.E16-06-0412. Epub 2017 May 3. Mol Biol Cell. 2017. PMID: 28468978 Free PMC article.
-
Grb2 interacts with SGEF and antagonizes the ability of SGEF to enhance EGF-induced ERK1/2 activation.Mol Cell Biochem. 2014 Apr;389(1-2):239-47. doi: 10.1007/s11010-013-1945-7. Epub 2014 Jan 8. Mol Cell Biochem. 2014. PMID: 24399467
-
Novel effect of the high risk-HPV E7 CKII phospho-acceptor site on polarity protein expression.BMC Cancer. 2022 Sep 25;22(1):1015. doi: 10.1186/s12885-022-10105-5. BMC Cancer. 2022. PMID: 36153517 Free PMC article.
-
Human tumour viruses and the deregulation of cell polarity in cancer.Nat Rev Cancer. 2012 Dec;12(12):877-86. doi: 10.1038/nrc3400. Nat Rev Cancer. 2012. PMID: 23175122 Review.
References
-
- zur Hausen H. Immortalization of human cells and their malignant conversion by high-risk human papillomavirus genotypes. Semin Cancer Biol. 1999;9:405–411. - PubMed
-
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. - PubMed
-
- Barbosa MS, Schlegel R. The E6 and E7 genes of HPV-18 are sufficient for inducing two-stage in vitro transformation of human keratinocytes. Oncogene. 1989;4:1529–1532. - PubMed
-
- Song S, Liem A, Miller JA, Lambert PF. Human papillomavirus type 16 E6 and E7 contribute differently to carcinogenesis. Virology. 2000;267:141–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

