Early priming minimizes the age-related immune compromise of CD8⁺ T cell diversity and function
- PMID: 22383879
- PMCID: PMC3285595
- DOI: 10.1371/journal.ppat.1002544
Early priming minimizes the age-related immune compromise of CD8⁺ T cell diversity and function
Erratum in
- PLoS Pathog. 2012 Mar;8(3). doi: 10.1371/annotation/e142f9de-7f30-4759-bda1-a651e86d5ba6
Abstract
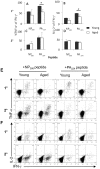
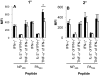
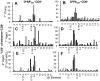
The elderly are particularly susceptible to influenza A virus infections, with increased occurrence, disease severity and reduced vaccine efficacy attributed to declining immunity. Experimentally, the age-dependent decline in influenza-specific CD8(+) T cell responsiveness reflects both functional compromise and the emergence of 'repertoire holes' arising from the loss of low frequency clonotypes. In this study, we asked whether early priming limits the time-related attrition of immune competence. Though primary responses in aged mice were compromised, animals vaccinated at 6 weeks then challenged >20 months later had T-cell responses that were normal in magnitude. Both functional quality and the persistence of 'preferred' TCR clonotypes that expand in a characteristic immunodominance hierarchy were maintained following early priming. Similar to the early priming, vaccination at 22 months followed by challenge retained a response magnitude equivalent to young mice. However, late priming resulted in reduced TCRβ diversity in comparison with vaccination earlier in life. Thus, early priming was critical to maintaining individual and population-wide TCRβ diversity. In summary, early exposure leads to the long-term maintenance of memory T cells and thus preserves optimal, influenza-specific CD8(+) T-cell responsiveness and protects against the age-related attrition of naïve T-cell precursors. Our study supports development of vaccines that prime CD8(+) T-cells early in life to elicit the broadest possible spectrum of CD8(+) T-cell memory and preserve the magnitude, functionality and TCR usage of responding populations. In addition, our study provides the most comprehensive analysis of the aged (primary, secondary primed-early and secondary primed-late) TCR repertoires published to date.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

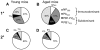



Similar articles
-
Homogenization of TCR repertoires within secondary CD62Lhigh and CD62Llow virus-specific CD8+ T cell populations.J Immunol. 2008 Jun 15;180(12):7938-47. doi: 10.4049/jimmunol.180.12.7938. J Immunol. 2008. PMID: 18523257
-
Perturbed CD8+ T cell immunity across universal influenza epitopes in the elderly.J Leukoc Biol. 2018 Feb;103(2):321-339. doi: 10.1189/jlb.5MA0517-207R. Epub 2017 Dec 29. J Leukoc Biol. 2018. PMID: 28928269
-
Effect of MHC class I diversification on influenza epitope-specific CD8+ T cell precursor frequency and subsequent effector function.J Immunol. 2011 Jun 1;186(11):6319-28. doi: 10.4049/jimmunol.1000883. Epub 2011 May 2. J Immunol. 2011. PMID: 21536802 Free PMC article.
-
Characterization of CD8+ T cell repertoire diversity and persistence in the influenza A virus model of localized, transient infection.Semin Immunol. 2004 Jun;16(3):179-84. doi: 10.1016/j.smim.2004.02.005. Semin Immunol. 2004. PMID: 15130502 Review.
-
Vaccine-Induced CD8+ T Cell Responses in Children: A Review of Age-Specific Molecular Determinants Contributing to Antigen Cross-Presentation.Front Immunol. 2020 Dec 23;11:607977. doi: 10.3389/fimmu.2020.607977. eCollection 2020. Front Immunol. 2020. PMID: 33424857 Free PMC article. Review.
Cited by
-
Testosterone treatment of aged male mice improves some but not all aspects of age-associated increases in influenza severity.Cell Immunol. 2019 Nov;345:103988. doi: 10.1016/j.cellimm.2019.103988. Epub 2019 Sep 14. Cell Immunol. 2019. PMID: 31540670 Free PMC article.
-
Reduced naïve CD8(+) T-cell priming efficacy in elderly adults.Aging Cell. 2016 Feb;15(1):14-21. doi: 10.1111/acel.12384. Epub 2015 Oct 15. Aging Cell. 2016. PMID: 26472076 Free PMC article.
-
Induction of memory cytotoxic T cells to influenza A virus and subsequent viral clearance is not modulated by PB1-F2-dependent inflammasome activation.Immunol Cell Biol. 2016 May;94(5):439-46. doi: 10.1038/icb.2015.115. Epub 2015 Dec 15. Immunol Cell Biol. 2016. PMID: 26667784 Free PMC article.
-
Tissue-resident CD8+ T cells drive age-associated chronic lung sequelae after viral pneumonia.Sci Immunol. 2020 Nov 6;5(53):eabc4557. doi: 10.1126/sciimmunol.abc4557. Sci Immunol. 2020. PMID: 33158975 Free PMC article.
-
Broad CD8+ T cell cross-recognition of distinct influenza A strains in humans.Nat Commun. 2018 Dec 21;9(1):5427. doi: 10.1038/s41467-018-07815-5. Nat Commun. 2018. PMID: 30575715 Free PMC article.
References
-
- Couch RB, Kasel JA, Glezen WP, Cate TR, Six HR, et al. Influenza: its control in persons and populations. J Infect Dis. 1986;153:431–440. - PubMed
-
- Webster RG. Immunity to influenza in the elderly. Vaccine. 2000;18:1686–1689. - PubMed
-
- LeMaoult J, Messaoudi I, Manavalan JS, Potvin H, Nikolich-Zugich D, et al. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. J Immunol. 2000;165:2367–2373. - PubMed
-
- Haynes L, Swain SL, Cambier J, Fulder R. Aging and immune function. Summary of a workshop held at Trudeau Institute, Saranac Lake, NY. Mech Ageing Dev. 2005;126:822–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

