Depletion of dendritic cells enhances innate anti-bacterial host defense through modulation of phagocyte homeostasis
- PMID: 22383883
- PMCID: PMC3285606
- DOI: 10.1371/journal.ppat.1002552
Depletion of dendritic cells enhances innate anti-bacterial host defense through modulation of phagocyte homeostasis
Abstract
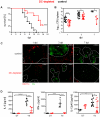
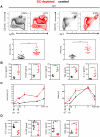
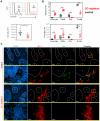
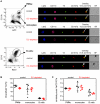
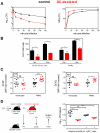
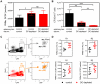
Dendritic cells (DCs) as professional antigen-presenting cells play an important role in the initiation and modulation of the adaptive immune response. However, their role in the innate immune response against bacterial infections is not completely defined. Here we have analyzed the role of DCs and their impact on the innate anti-bacterial host defense in an experimental infection model of Yersinia enterocolitica (Ye). We used CD11c-diphtheria toxin (DT) mice to deplete DCs prior to severe infection with Ye. DC depletion significantly increased animal survival after Ye infection. The bacterial load in the spleen of DC-depleted mice was significantly lower than that of control mice throughout the infection. DC depletion was accompanied by an increase in the serum levels of CXCL1, G-CSF, IL-1α, and CCL2 and an increase in the numbers of splenic phagocytes. Functionally, splenocytes from DC-depleted mice exhibited an increased bacterial killing capacity compared to splenocytes from control mice. Cellular studies further showed that this was due to an increased production of reactive oxygen species (ROS) by neutrophils. Adoptive transfer of neutrophils from DC-depleted mice into control mice prior to Ye infection reduced the bacterial load to the level of Ye-infected DC-depleted mice, suggesting that the increased number of phagocytes with additional ROS production account for the decreased bacterial load. Furthermore, after incubation with serum from DC-depleted mice splenocytes from control mice increased their bacterial killing capacity, most likely due to enhanced ROS production by neutrophils, indicating that serum factors from DC-depleted mice account for this effect. In summary, we could show that DC depletion triggers phagocyte accumulation in the spleen and enhances their anti-bacterial killing capacity upon bacterial infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. - PubMed
-
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. - PubMed
-
- Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Bell D, Young JW, Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

