A P-loop mutation in Gα subunits prevents transition to the active state: implications for G-protein signaling in fungal pathogenesis
- PMID: 22383884
- PMCID: PMC3285607
- DOI: 10.1371/journal.ppat.1002553
A P-loop mutation in Gα subunits prevents transition to the active state: implications for G-protein signaling in fungal pathogenesis
Abstract
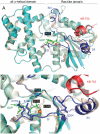
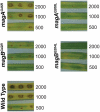
Heterotrimeric G-proteins are molecular switches integral to a panoply of different physiological responses that many organisms make to environmental cues. The switch from inactive to active Gαβγ heterotrimer relies on nucleotide cycling by the Gα subunit: exchange of GTP for GDP activates Gα, whereas its intrinsic enzymatic activity catalyzes GTP hydrolysis to GDP and inorganic phosphate, thereby reverting Gα to its inactive state. In several genetic studies of filamentous fungi, such as the rice blast fungus Magnaporthe oryzae, a G42R mutation in the phosphate-binding loop of Gα subunits is assumed to be GTPase-deficient and thus constitutively active. Here, we demonstrate that Gα(G42R) mutants are not GTPase deficient, but rather incapable of achieving the activated conformation. Two crystal structure models suggest that Arg-42 prevents a typical switch region conformational change upon Gα(i1)(G42R) binding to GDP·AlF(4)(-) or GTP, but rotameric flexibility at this locus allows for unperturbed GTP hydrolysis. Gα(G42R) mutants do not engage the active state-selective peptide KB-1753 nor RGS domains with high affinity, but instead favor interaction with Gβγ and GoLoco motifs in any nucleotide state. The corresponding Gα(q)(G48R) mutant is not constitutively active in cells and responds poorly to aluminum tetrafluoride activation. Comparative analyses of M. oryzae strains harboring either G42R or GTPase-deficient Q/L mutations in the Gα subunits MagA or MagB illustrate functional differences in environmental cue processing and intracellular signaling outcomes between these two Gα mutants, thus demonstrating the in vivo functional divergence of G42R and activating G-protein mutants.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Rgs1 regulates multiple Galpha subunits in Magnaporthe pathogenesis, asexual growth and thigmotropism.EMBO J. 2007 Feb 7;26(3):690-700. doi: 10.1038/sj.emboj.7601536. Epub 2007 Jan 25. EMBO J. 2007. PMID: 17255942 Free PMC article.
-
Site-directed mutagenesis of the magB gene affects growth and development in Magnaporthe grisea.Mol Plant Microbe Interact. 2000 Nov;13(11):1214-27. doi: 10.1094/MPMI.2000.13.11.1214. Mol Plant Microbe Interact. 2000. PMID: 11059488
-
The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits.Int J Biol Sci. 2005;1(2):51-66. doi: 10.7150/ijbs.1.51. Epub 2005 Apr 1. Int J Biol Sci. 2005. PMID: 15951850 Free PMC article. Review.
-
Invited review: Activation of G proteins by GTP and the mechanism of Gα-catalyzed GTP hydrolysis.Biopolymers. 2016 Aug;105(8):449-62. doi: 10.1002/bip.22836. Biopolymers. 2016. PMID: 26996924 Free PMC article. Review.
-
Integration of G protein α (Gα) signaling by the regulator of G protein signaling 14 (RGS14).J Biol Chem. 2015 Apr 3;290(14):9037-49. doi: 10.1074/jbc.M114.634329. Epub 2015 Feb 9. J Biol Chem. 2015. PMID: 25666614 Free PMC article.
Cited by
-
Directly light-regulated binding of RGS-LOV photoreceptors to anionic membrane phospholipids.Proc Natl Acad Sci U S A. 2018 Aug 14;115(33):E7720-E7727. doi: 10.1073/pnas.1802832115. Epub 2018 Jul 31. Proc Natl Acad Sci U S A. 2018. PMID: 30065115 Free PMC article.
-
Genetic modeling of GNAO1 disorder delineates mechanisms of Gαo dysfunction.Hum Mol Genet. 2022 Feb 21;31(4):510-522. doi: 10.1093/hmg/ddab235. Hum Mol Genet. 2022. PMID: 34508586 Free PMC article.
-
Heterotrimeric G-protein signaling is critical to pathogenic processes in Entamoeba histolytica.PLoS Pathog. 2012;8(11):e1003040. doi: 10.1371/journal.ppat.1003040. Epub 2012 Nov 15. PLoS Pathog. 2012. PMID: 23166501 Free PMC article.
-
Structure-function analysis of Rgs1 in Magnaporthe oryzae: role of DEP domains in subcellular targeting.PLoS One. 2012;7(7):e41084. doi: 10.1371/journal.pone.0041084. Epub 2012 Jul 19. PLoS One. 2012. PMID: 22927898 Free PMC article.
-
System-Wide Characterization of MoArf GTPase Family Proteins and Adaptor Protein MoGga1 Involved in the Development and Pathogenicity of Magnaporthe oryzae.mBio. 2019 Oct 15;10(5):e02398-19. doi: 10.1128/mBio.02398-19. mBio. 2019. PMID: 31615964 Free PMC article.
References
-
- Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, et al. The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell. 1995;83:1047–1058. - PubMed
-
- Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, et al. Structures of active conformations of Gi alpha 1 and the mechanism of GTP hydrolysis. Science. 1994;265:1405–1412. - PubMed
-
- Mazzoni MR, Hamm HE. Interaction of transducin with light-activated rhodopsin protects it from proteolytic digestion by trypsin. J Biol Chem. 1996;271:30034–30040. - PubMed
-
- Higashijima T, Ferguson KM, Sternweis PC, Ross EM, Smigel MD, et al. The effect of activating ligands on the intrinsic fluorescence of guanine nucleotide-binding regulatory proteins. J Biol Chem. 1987;262:752–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

