Evaluation of Measles Vaccine Virus as a Vector to Deliver Respiratory Syncytial Virus Fusion Protein or Epstein-Barr Virus Glycoprotein gp350
- PMID: 22383906
- PMCID: PMC3286841
- DOI: 10.2174/1874357901206010012
Evaluation of Measles Vaccine Virus as a Vector to Deliver Respiratory Syncytial Virus Fusion Protein or Epstein-Barr Virus Glycoprotein gp350
Abstract
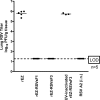
Live attenuated recombinant measles vaccine virus (MV) Edmonston-Zagreb (EZ) strain was evaluated as a viral vector to express the ectodomains of fusion protein of respiratory syncytial virus (RSV F) or glycoprotein 350 of Epstein-Barr virus (EBV gp350) as candidate vaccines for prophylaxis of RSV and EBV. The glycoprotein gene was inserted at the 1(st) or the 3(rd) position of the measles virus genome and the recombinant viruses were generated. Insertion of the foreign gene at the 3(rd) position had a minimal impact on viral replication in vitro. RSV F or EBV gp350 protein was secreted from infected cells. In cotton rats, EZ-RSV F and EZ-EBV gp350 induced MV- and insert-specific antibody responses. In addition, both vaccines also induced insert specific interferon gamma (IFN-γ) secreting T cell response. EZ-RSV F protected cotton rats from pulmonary replication of RSV A2 challenge infection. In rhesus macaques, although both EZ-RSV F and EZ-EBV gp350 induced MV specific neutralizing antibody responses, only RSV F specific antibody response was detected. Thus, the immunogenicity of the foreign antigens delivered by measles vaccine virus is dependent on the nature of the insert and the animal models used for vaccine evaluation.
Keywords: Cotton rats; EBV gp350; Edmonston-Zagreb; Measles vaccine; RSV F; Rhesus monkeys..
Figures

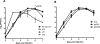

Similar articles
-
Murine Pneumonia Virus Expressing the Fusion Glycoprotein of Human Respiratory Syncytial Virus from an Added Gene Is Highly Attenuated and Immunogenic in Rhesus Macaques.J Virol. 2018 Aug 16;92(17):e00723-18. doi: 10.1128/JVI.00723-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925656 Free PMC article.
-
A Single-Dose Recombinant Parainfluenza Virus 5-Vectored Vaccine Expressing Respiratory Syncytial Virus (RSV) F or G Protein Protected Cotton Rats and African Green Monkeys from RSV Challenge.J Virol. 2017 May 12;91(11):e00066-17. doi: 10.1128/JVI.00066-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28298602 Free PMC article.
-
Epstein-Barr Virus gp350 Can Functionally Replace the Rhesus Lymphocryptovirus Major Membrane Glycoprotein and Does Not Restrict Infection of Rhesus Macaques.J Virol. 2015 Nov 11;90(3):1222-30. doi: 10.1128/JVI.02531-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26559839 Free PMC article.
-
Four Decades of Prophylactic EBV Vaccine Research: A Systematic Review and Historical Perspective.Front Immunol. 2022 Apr 14;13:867918. doi: 10.3389/fimmu.2022.867918. eCollection 2022. Front Immunol. 2022. PMID: 35493498 Free PMC article.
-
Urgency and necessity of Epstein-Barr virus prophylactic vaccines.NPJ Vaccines. 2022 Dec 9;7(1):159. doi: 10.1038/s41541-022-00587-6. NPJ Vaccines. 2022. PMID: 36494369 Free PMC article. Review.
Cited by
-
Live-attenuated YF17D-vectored COVID-19 vaccine protects from lethal yellow fever virus infection in mouse and hamster models.EBioMedicine. 2022 Sep;83:104240. doi: 10.1016/j.ebiom.2022.104240. Epub 2022 Aug 27. EBioMedicine. 2022. PMID: 36041265 Free PMC article.
-
A chimeric EBV gp350/220-based VLP replicates the virion B-cell attachment mechanism and elicits long-lasting neutralizing antibodies in mice.J Transl Med. 2015 Feb 6;13:50. doi: 10.1186/s12967-015-0415-2. J Transl Med. 2015. PMID: 25885535 Free PMC article.
-
Recombinant measles AIK-C vaccine strain expressing heterologous virus antigens.Vaccine. 2016 Jan 4;34(2):292-295. doi: 10.1016/j.vaccine.2015.10.127. Epub 2015 Nov 10. Vaccine. 2016. PMID: 26562316 Free PMC article. Review.
-
Engineering and combining oncolytic measles virus for cancer therapy.Cytokine Growth Factor Rev. 2020 Dec;56:39-48. doi: 10.1016/j.cytogfr.2020.07.005. Epub 2020 Jul 3. Cytokine Growth Factor Rev. 2020. PMID: 32718830 Free PMC article. Review.
-
Development of Recombinant Measles Virus-Based Vaccines.Methods Mol Biol. 2017;1581:151-168. doi: 10.1007/978-1-4939-6869-5_9. Methods Mol Biol. 2017. PMID: 28374248 Free PMC article.
References
-
- Liniger M, Zuniga A, Naim HY. Use of viral vectors for the development of vaccines. Expert Rev Vaccines. 2007;6(2 ):255–66. - PubMed
-
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19 ):1903–15. - PubMed
-
- Gans HA, Yasukawa LL, Alderson A, et al. T cell immunity to measles viral proteins in infants and adults after measles immunization. Viral Immunol. 2004;17(2 ):298–307. - PubMed
-
- Hiremath GS, Omer SB. A meta-analysis of studies comparing the respiratory route with the subcutaneous route of measles vaccine administration. Hum Vaccin. 2005;1(1 ):30–6. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
