Respiratory syncytial virus NS1 protein colocalizes with mitochondrial antiviral signaling protein MAVS following infection
- PMID: 22383950
- PMCID: PMC3288005
- DOI: 10.1371/journal.pone.0029386
Respiratory syncytial virus NS1 protein colocalizes with mitochondrial antiviral signaling protein MAVS following infection
Abstract
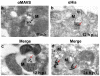
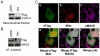
Respiratory syncytial virus (RSV) nonstructural protein 1(NS1) attenuates type-I interferon (IFN) production during RSV infection; however the precise role of RSV NS1 protein in orchestrating the early host-virus interaction during infection is poorly understood. Since NS1 constitutes the first RSV gene transcribed and the production of IFN depends upon RLR (RIG-I-like receptor) signaling, we reasoned that NS1 may interfere with this signaling. Herein, we report that NS1 is localized to mitochondria and binds to mitochondrial antiviral signaling protein (MAVS). Live-cell imaging of rgRSV-infected A549 human epithelial cells showed that RSV replication and transcription occurs in proximity to mitochondria. NS1 localization to mitochondria was directly visualized by confocal microscopy using a cell-permeable chemical probe for His(6)-NS1. Further, NS1 colocalization with MAVS in A549 cells infected with RSV was shown by confocal laser microscopy and immuno-electron microscopy. NS1 protein is present in the mitochondrial fraction and co-immunoprecipitates with MAVS in total cell lysatesof A549 cells transfected with the plasmid pNS1-Flag. By immunoprecipitation with anti-RIG-I antibody, RSV NS1 was shown to associate with MAVS at an early stage of RSV infection, and to disrupt MAVS interaction with RIG-I (retinoic acid inducible gene) and the downstream IFN antiviral and inflammatory response. Together, these results demonstrate that NS1 binds to MAVS and that this binding inhibits the MAVS-RIG-I interaction required for IFN production.
Conflict of interest statement
Figures




Similar articles
-
Human Respiratory Syncytial Virus NS 1 Targets TRIM25 to Suppress RIG-I Ubiquitination and Subsequent RIG-I-Mediated Antiviral Signaling.Viruses. 2018 Dec 14;10(12):716. doi: 10.3390/v10120716. Viruses. 2018. PMID: 30558248 Free PMC article.
-
Subcellular Localizations of RIG-I, TRIM25, and MAVS Complexes.J Virol. 2017 Jan 3;91(2):e01155-16. doi: 10.1128/JVI.01155-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807226 Free PMC article.
-
Leukemia inhibitory factor protects the lung during respiratory syncytial viral infection.BMC Immunol. 2014 Oct 3;15:41. doi: 10.1186/s12865-014-0041-4. BMC Immunol. 2014. PMID: 25277705 Free PMC article.
-
An Unexpected Encounter: Respiratory Syncytial Virus Nonstructural Protein 1 Interacts with Mediator Subunit MED25.J Virol. 2022 Oct 12;96(19):e0129722. doi: 10.1128/jvi.01297-22. Epub 2022 Sep 14. J Virol. 2022. PMID: 36102648 Free PMC article. Review.
-
Respiratory syncytial virus nonstructural proteins 1 and 2: Exceptional disrupters of innate immune responses.PLoS Pathog. 2019 Oct 17;15(10):e1007984. doi: 10.1371/journal.ppat.1007984. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31622448 Free PMC article. Review.
Cited by
-
Structural basis for IFN antagonism by human respiratory syncytial virus nonstructural protein 2.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2020587118. doi: 10.1073/pnas.2020587118. Proc Natl Acad Sci U S A. 2021. PMID: 33649232 Free PMC article.
-
Host cytoskeleton in respiratory syncytial virus assembly and budding.Virol J. 2016 Sep 26;13(1):161. doi: 10.1186/s12985-016-0618-z. Virol J. 2016. PMID: 27670781 Free PMC article. Review.
-
Biology of Infection and Disease Pathogenesis to Guide RSV Vaccine Development.Front Immunol. 2019 Jul 25;10:1675. doi: 10.3389/fimmu.2019.01675. eCollection 2019. Front Immunol. 2019. PMID: 31402910 Free PMC article. Review.
-
Role of mitochondria in parvovirus pathology.PLoS One. 2014 Jan 21;9(1):e86124. doi: 10.1371/journal.pone.0086124. eCollection 2014. PLoS One. 2014. PMID: 24465910 Free PMC article.
-
Genetic and Epigenetic Host-Virus Network to Investigate Pathogenesis and Identify Biomarkers for Drug Repurposing of Human Respiratory Syncytial Virus via Real-World Two-Side RNA-Seq Data: Systems Biology and Deep-Learning Approach.Biomedicines. 2023 May 25;11(6):1531. doi: 10.3390/biomedicines11061531. Biomedicines. 2023. PMID: 37371627 Free PMC article.
References
-
- Wong DT, Ogra PL. Viral infections in immunocompromised patients. Med Clin North Am. 1983;67:1075–1092. - PubMed
-
- Ebbert JO, Limper AH. Respiratory Syncytial Virus Pneumonitis in Immunocompromised Adults: Clinical Features and Outcome. Respiration. 2005;72:263–269. - PubMed
-
- Blanchard SS, Gerrek M, Siegel C, Czinn SJ. Significant morbidity associated with RSV infection in immunosuppressed children following liver transplantation: Case report and discussion regarding need of routine prophylaxis. Pediatric Transplantation. 2006;10:826–829. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. - PubMed
-
- Becker Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy - a review. Virus Genes. 2006;33:235–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

