Circadian rhythms of fetal liver transcription persist in the absence of canonical circadian clock gene expression rhythms in vivo
- PMID: 22383974
- PMCID: PMC3285613
- DOI: 10.1371/journal.pone.0030781
Circadian rhythms of fetal liver transcription persist in the absence of canonical circadian clock gene expression rhythms in vivo
Abstract
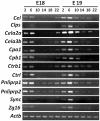
The cellular circadian clock and systemic cues drive rhythmicity in the transcriptome of adult peripheral tissues. However, the oscillating status of the circadian clocks in fetal tissues, and their response to maternal cues, are less clear. Most clock genes do not cycle in fetal livers from mice and rats, although tissue level rhythms rapidly emerge when fetal mouse liver explants are cultured in vitro. Thus, in the fetal mouse liver, the circadian clock does not oscillate at the cellular level (but is induced to oscillate in culture). To gain a comprehensive overview of the clock status in the fetal liver during late gestation, we performed microarray analyses on fetal liver tissues. In the fetal liver we did not observe circadian rhythms of clock gene expression or many other transcripts known to be rhythmically expressed in the adult liver. Nevertheless, JTK_CYCLE analysis identified some transcripts in the fetal liver that were rhythmically expressed, albeit at low amplitudes. Upon data filtering by coefficient of variation, the expression levels for transcripts related to pancreatic exocrine enzymes and zymogen secretion were found to undergo synchronized daily fluctuations at high amplitudes. These results suggest that maternal cues influence the fetal liver, despite the fact that we did not detect circadian rhythms of canonical clock gene expression in the fetal liver. These results raise important questions on the role of the circadian clock, or lack thereof, during ontogeny.
Conflict of interest statement
Figures



Similar articles
-
Transcriptome comparison between fetal and adult mouse livers: implications for circadian clock mechanisms.PLoS One. 2012;7(2):e31292. doi: 10.1371/journal.pone.0031292. Epub 2012 Feb 21. PLoS One. 2012. PMID: 22363607 Free PMC article.
-
Embryonic development and maternal regulation of murine circadian clock function.Chronobiol Int. 2015 Apr;32(3):416-27. doi: 10.3109/07420528.2014.986576. Epub 2014 Nov 28. Chronobiol Int. 2015. PMID: 25431080
-
Rewiring of liver diurnal transcriptome rhythms by triiodothyronine (T3) supplementation.Elife. 2022 Jul 27;11:e79405. doi: 10.7554/eLife.79405. Elife. 2022. PMID: 35894384 Free PMC article.
-
Circadian clocks during embryonic and fetal development.Birth Defects Res C Embryo Today. 2007 Sep;81(3):204-14. doi: 10.1002/bdrc.20101. Birth Defects Res C Embryo Today. 2007. PMID: 17963275 Review.
-
Genetics and molecular biology of rhythms in Drosophila and other insects.Adv Genet. 2003;48:1-280. doi: 10.1016/s0065-2660(03)48000-0. Adv Genet. 2003. PMID: 12593455 Review.
Cited by
-
Clocks underneath: the role of peripheral clocks in the timing of female reproductive physiology.Front Endocrinol (Lausanne). 2013 Jul 23;4:91. doi: 10.3389/fendo.2013.00091. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23888155 Free PMC article.
-
Sex Inclusion in Transcriptome Studies of Daily Rhythms.J Biol Rhythms. 2023 Feb;38(1):3-14. doi: 10.1177/07487304221134160. Epub 2022 Nov 23. J Biol Rhythms. 2023. PMID: 36419398 Free PMC article.
References
-
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. - PubMed
-
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. - PubMed
-
- Ukai H, Ueda HR. Systems biology of mammalian circadian clocks. Annu Rev Physiol. 2010;72:579–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

