A mathematical model of mitotic exit in budding yeast: the role of Polo kinase
- PMID: 22383977
- PMCID: PMC3285609
- DOI: 10.1371/journal.pone.0030810
A mathematical model of mitotic exit in budding yeast: the role of Polo kinase
Abstract
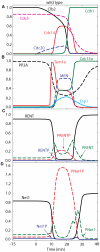
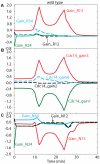
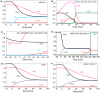
Cell cycle progression in eukaryotes is regulated by periodic activation and inactivation of a family of cyclin-dependent kinases (Cdk's). Entry into mitosis requires phosphorylation of many proteins targeted by mitotic Cdk, and exit from mitosis requires proteolysis of mitotic cyclins and dephosphorylation of their targeted proteins. Mitotic exit in budding yeast is known to involve the interplay of mitotic kinases (Cdk and Polo kinases) and phosphatases (Cdc55/PP2A and Cdc14), as well as the action of the anaphase promoting complex (APC) in degrading specific proteins in anaphase and telophase. To understand the intricacies of this mechanism, we propose a mathematical model for the molecular events during mitotic exit in budding yeast. The model captures the dynamics of this network in wild-type yeast cells and 110 mutant strains. The model clarifies the roles of Polo-like kinase (Cdc5) in the Cdc14 early anaphase release pathway and in the G-protein regulated mitotic exit network.
Conflict of interest statement
Figures
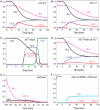
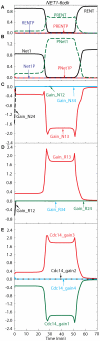
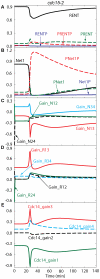
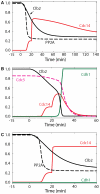

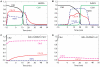
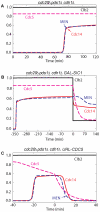
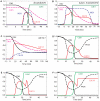
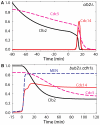
Similar articles
-
Mitotic exit in two dimensions.J Theor Biol. 2007 Oct 7;248(3):560-73. doi: 10.1016/j.jtbi.2007.06.014. Epub 2007 Jun 17. J Theor Biol. 2007. PMID: 17659305
-
Oscillations in Cdc14 release and sequestration reveal a circuit underlying mitotic exit.J Cell Biol. 2010 Jul 26;190(2):209-22. doi: 10.1083/jcb.201002026. J Cell Biol. 2010. PMID: 20660629 Free PMC article.
-
APC/C-Cdh1-mediated degradation of the Polo kinase Cdc5 promotes the return of Cdc14 into the nucleolus.Genes Dev. 2008 Jan 1;22(1):79-90. doi: 10.1101/gad.1601308. Genes Dev. 2008. PMID: 18172166 Free PMC article.
-
Complexity of mitotic exit.Cell Cycle. 2002 Sep-Oct;1(5):300-3. doi: 10.4161/cc.1.5.142. Cell Cycle. 2002. PMID: 12461287 Review.
-
Polo-like kinase 1: target and regulator of anaphase-promoting complex/cyclosome-dependent proteolysis.Cancer Res. 2006 Jul 15;66(14):6895-8. doi: 10.1158/0008-5472.CAN-06-0358. Cancer Res. 2006. PMID: 16849530 Review.
Cited by
-
Principles, mechanisms and functions of entrainment in biological oscillators.Interface Focus. 2022 Apr 15;12(3):20210088. doi: 10.1098/rsfs.2021.0088. eCollection 2022 Jun 6. Interface Focus. 2022. PMID: 35450280 Free PMC article. Review.
-
Model-Based Analysis of Cell Cycle Responses to Dynamically Changing Environments.PLoS Comput Biol. 2016 Jan 7;12(1):e1004604. doi: 10.1371/journal.pcbi.1004604. eCollection 2016 Jan. PLoS Comput Biol. 2016. PMID: 26741131 Free PMC article.
-
Bistability, oscillations, and traveling waves in frog egg extracts.Bull Math Biol. 2015 May;77(5):796-816. doi: 10.1007/s11538-014-0009-9. Epub 2014 Sep 4. Bull Math Biol. 2015. PMID: 25185750 Free PMC article.
-
Cdc14 Early Anaphase Release, FEAR, Is Limited to the Nucleus and Dispensable for Efficient Mitotic Exit.PLoS One. 2015 Jun 19;10(6):e0128604. doi: 10.1371/journal.pone.0128604. eCollection 2015. PLoS One. 2015. PMID: 26090959 Free PMC article.
-
The FEAR protein Slk19 restricts Cdc14 phosphatase to the nucleus until the end of anaphase, regulating its participation in mitotic exit in Saccharomyces cerevisiae.PLoS One. 2013 Sep 10;8(9):e73194. doi: 10.1371/journal.pone.0073194. eCollection 2013. PLoS One. 2013. PMID: 24039885 Free PMC article.
References
-
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. - PubMed
-
- Novak B, Tyson JJ. Quantitative-Analysis of a Molecular-Model of Mitotic Control in Fission Yeast. Journal of Theoretical Biology. 1995;173:283–305.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

