Dilated thin-walled blood and lymphatic vessels in human endometrium: a potential role for VEGF-D in progestin-induced break-through bleeding
- PMID: 22383980
- PMCID: PMC3284580
- DOI: 10.1371/journal.pone.0030916
Dilated thin-walled blood and lymphatic vessels in human endometrium: a potential role for VEGF-D in progestin-induced break-through bleeding
Erratum in
-
Correction: Dilated Thin-Walled Blood and Lymphatic Vessels in Human Endometrium: A Potential Role for VEGF-D in Progestin-Induced Break-Through Bleeding.PLoS One. 2021 Oct 26;16(10):e0259337. doi: 10.1371/journal.pone.0259337. eCollection 2021. PLoS One. 2021. PMID: 34699577 Free PMC article.
Abstract
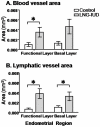
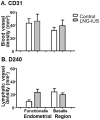
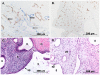
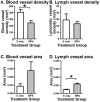
Progestins provide safe, effective and cheap options for contraception as well as the treatment of a variety of gynaecological disorders. Episodes of irregular endometrial bleeding or breakthrough bleeding (BTB) are a major unwanted side effect of progestin treatment, such that BTB is the leading cause for discontinued use of an otherwise effective and popular medication. The cellular mechanisms leading to BTB are poorly understood. In this study, we make the novel finding that the large, dilated, thin walled vessels characteristic of human progestin-treated endometrium include both blood and lymphatic vessels. Increased blood and lymphatic vessel diameter are features of VEGF-D action in other tissues and we show by immunolocalisation and Western blotting that stromal cell decidualisation results in a significant increase in VEGF-D protein production, particularly of the proteolytically processed 21 kD form. Using a NOD/scid mouse model with xenografted human endometrium we were able to show that progestin treatment causes decidualisation, VEGF-D production and endometrial vessel dilation. Our results lead to a novel hypothesis to explain BTB, with stromal cell decidualisation rather than progestin treatment per se being the proposed causative event, and VEGF-D being the proposed effector agent.
Conflict of interest statement
Figures




Similar articles
-
Endometrial vasculature in Norplant users.Hum Reprod. 1996 Oct;11 Suppl 2:45-50. doi: 10.1093/humrep/11.suppl_2.45. Hum Reprod. 1996. PMID: 8982745
-
Lymphangiogenesis of normal endometrium and endometrial adenocarcinoma.Hum Reprod. 2007 Jun;22(6):1705-13. doi: 10.1093/humrep/dem037. Epub 2007 Mar 8. Hum Reprod. 2007. PMID: 17347164
-
Progestin-regulated expression of tissue factor in decidual cells: implications in endometrial hemostasis, menstruation and angiogenesis.Steroids. 2003 Nov;68(10-13):849-60. doi: 10.1016/s0039-128x(03)00139-9. Steroids. 2003. PMID: 14667977 Review.
-
Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery.Hum Reprod. 1998 May;13(5):1218-24. doi: 10.1093/humrep/13.5.1218. Hum Reprod. 1998. PMID: 9647550
-
Angiogenic factors and the endometrium following long term progestin only contraception.Histol Histopathol. 2004 Jan;19(1):167-72. doi: 10.14670/HH-19.167. Histol Histopathol. 2004. PMID: 14702185 Review.
Cited by
-
The impact of combined oral contraceptives on ocular tissues: a review of ocular effects.Int J Ophthalmol. 2017 Oct 18;10(10):1604-1610. doi: 10.18240/ijo.2017.10.19. eCollection 2017. Int J Ophthalmol. 2017. PMID: 29062782 Free PMC article. Review.
-
Pre-vascularization Enhances Therapeutic Effects of Human Mesenchymal Stem Cell Sheets in Full Thickness Skin Wound Repair.Theranostics. 2017 Jan 1;7(1):117-131. doi: 10.7150/thno.17031. eCollection 2017. Theranostics. 2017. PMID: 28042321 Free PMC article.
-
Endometrial Angiogenesis of Abnormal Uterine Bleeding and Infertility in Patients with Uterine Fibroids-A Systematic Review.Int J Mol Sci. 2023 Apr 10;24(8):7011. doi: 10.3390/ijms24087011. Int J Mol Sci. 2023. PMID: 37108180 Free PMC article.
-
Lymphatic Vessel Network Structure and Physiology.Compr Physiol. 2018 Dec 13;9(1):207-299. doi: 10.1002/cphy.c180015. Compr Physiol. 2018. PMID: 30549020 Free PMC article. Review.
-
Evolution of the Systems Pharmacokinetics-Pharmacodynamics Model for Antibody-Drug Conjugates to Characterize Tumor Heterogeneity and In Vivo Bystander Effect.J Pharmacol Exp Ther. 2020 Jul;374(1):184-199. doi: 10.1124/jpet.119.262287. Epub 2020 Apr 9. J Pharmacol Exp Ther. 2020. PMID: 32273304 Free PMC article.
References
-
- Rodriguez MI, Warden M, Darney PD. Intrauterine progestins, progesterone antagonists, and receptor modulators: a review of gynecologic applications. Am J Obstet Gynecol. 2010;202:420–428. - PubMed
-
- Stewart A, Cummins C, Gold L, Jordan R, Phillips W. The effectiveness of the levonorgestrel-releasing intrauterine system in menorrhagia: a systematic review. BJOG. 2001;108:74–86. - PubMed
-
- Rodgers AK, Falcone T. Treatment strategies for endometriosis. Expert Opin Pharmacother. 2008;9:243–255. - PubMed
-
- d'Arcangues C. Management of vaginal bleeding irregularities induced by progestin-only contraceptives. Hum Reprod. 2000;15(Suppl 3):24–29. - PubMed
-
- Findlay JK. Future directions for research on endometrial bleeding. Hum Reprod. 1996;11(Suppl 2):179–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

