Molecular characterization, tissue distribution, subcellular localization and actin-sequestering function of a thymosin protein from silkworm
- PMID: 22383992
- PMCID: PMC3284464
- DOI: 10.1371/journal.pone.0031040
Molecular characterization, tissue distribution, subcellular localization and actin-sequestering function of a thymosin protein from silkworm
Abstract
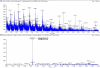
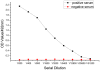
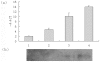
We identified a novel gene encoding a Bombyx mori thymosin (BmTHY) protein from a cDNA library of silkworm pupae, which has an open reading frame (ORF) of 399 bp encoding 132 amino acids. It was found by bioinformatics that BmTHY gene consisted of three exons and two introns and BmTHY was highly homologous to thymosin betas (Tβ). BmTHY has a conserved motif LKHTET with only one amino acid difference from LKKTET, which is involved in Tβ binding to actin. A His-tagged BmTHY fusion protein (rBmTHY) with a molecular weight of approximately 18.4 kDa was expressed and purified to homogeneity. The purified fusion protein was used to produce anti-rBmTHY polyclonal antibodies in a New Zealand rabbit. Subcellular localization revealed that BmTHY can be found in both Bm5 cell (a silkworm ovary cell line) nucleus and cytoplasm but is primarily located in the nucleus. Western blotting and real-time RT-PCR showed that during silkworm developmental stages, BmTHY expression levels are highest in moth, followed by instar larvae, and are lowest in pupa and egg. BmTHY mRNA was universally distributed in most of fifth-instar larvae tissues (except testis). However, BmTHY was expressed in the head, ovary and epidermis during the larvae stage. BmTHY formed complexes with actin monomer, inhibited actin polymerization and cross-linked to actin. All the results indicated BmTHY might be an actin-sequestering protein and participate in silkworm development.
Conflict of interest statement
Figures








References
-
- Turrini P, Tirassa P, Vigneti E, Aloe L. A role of the thymus and thymosin-alpha1 in brain NGF levels and NGF receptor expression. J Neuroimmunol. 1998;82:64–72. - PubMed
-
- Hadden JW. Thymic endocrinology. Int J Immunopharmacol. 1992;14:345–352. - PubMed
-
- Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Review of thymic hormones in cancer diagnosis and treatment. Int J Immunopharmacol. 2000;22:261–273. - PubMed
-
- Windmill KF, Lee VW. Influences of surgical castration on the thymus of male rats. J Reprod Immunol. 1999;44:29–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

