In vitro study of novel collagenase (XIAFLEX®) on Dupuytren's disease fibroblasts displays unique drug related properties
- PMID: 22384021
- PMCID: PMC3286458
- DOI: 10.1371/journal.pone.0031430
In vitro study of novel collagenase (XIAFLEX®) on Dupuytren's disease fibroblasts displays unique drug related properties
Abstract
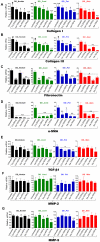
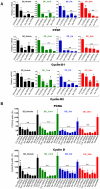
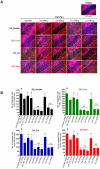
Dupuytren's disease (DD) is a benign, fibroproliferative disease of the palmar fascia, with excessive extracellular matrix (ECM) deposition and over-production of cytokines and growth factors, resulting in digital fixed flexion contractures limiting hand function and patient quality of life. Surgical fasciectomy is the gold standard treatment but is invasive and has associated morbidity without limiting disease recurrence. Injectable Collagenase Clostridium histolyticum (CCH)--Xiaflex®--is a novel, nonsurgical option with clinically proven in vivo reduction of DD contractures but with limited in vitro data demonstrating its cellular and molecular effects. The aim of this study was to delineate the effects of CCH on primary fibroblasts isolated from DD and non-DD anatomical sites (using RTCA, LDH, WST-1, FACS, qRT-PCR, ELISA and In-Cell Quantitative Western Blotting) to compare the efficacy of varying concentrations of Xiaflex® against a reagent grade Collagenase, Collagenase A. Results demonstrated that DD nodule and cord fibroblasts had greater proliferation than those from fat and skin. Xiaflex® exposure resulted in dose- and time-dependent inhibition of cellular spreading, attachment and proliferation, with cellular recovery after enzyme removal. Unlike Collagenase A, Xiaflex® did not cause apoptosis. Collagen expression patterns were significantly (p<0.05) different in DD fibroblasts across anatomical sites - the highest levels of collagen I and III were detected in DD nodule, with DD cord and fat fibroblasts demonstrating a smaller increase in both collagen expression relative to DD skin. Xiaflex® significantly (p<0.05) down-regulated ECM components, cytokines and growth factors in a dose-dependent manner. An in vitro scratch wound assay model demonstrated that, at low concentrations, Xiaflex® enabled a faster fibroblast reparatory migration into the wound, whereas, at high concentrations, this process was significantly (p<0.05) inhibited. This is the first report elucidating potential mechanisms of action of Xiaflex® on Dupuytren fibroblasts, offering a greater insight and a better understanding of its effect in DD.
Conflict of interest statement
Figures
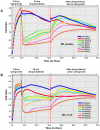

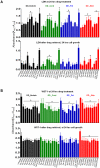
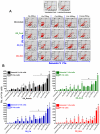



Similar articles
-
Dupuytren's Contracture Recurrence and Treatment Following Collagenase Clostridium Histolyticum Injection: A Longitudinal Assessment in a Veteran Population.Mil Med. 2023 Aug 29;188(9-10):e2975-e2981. doi: 10.1093/milmed/usad075. Mil Med. 2023. PMID: 36928340
-
Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren's disease.J Hand Surg Am. 2010 Dec;35(12):2027-38.e1. doi: 10.1016/j.jhsa.2010.08.007. J Hand Surg Am. 2010. PMID: 21134613 Clinical Trial.
-
Anti-fibrotic action of pirfenidone in Dupuytren's disease-derived fibroblasts.BMC Musculoskelet Disord. 2016 Nov 11;17(1):469. doi: 10.1186/s12891-016-1326-y. BMC Musculoskelet Disord. 2016. PMID: 27835939 Free PMC article.
-
Collagenase clostridium histolyticum injection for the treatment of Dupuytren's contracture.Drugs Today (Barc). 2011 Sep;47(9):653-67. doi: 10.1358/dot.2011.47.9.1656502. Drugs Today (Barc). 2011. PMID: 21971540 Review.
-
[Collagenase Clostridum histolyticum in the management of Dupuytren's contracture].Handchir Mikrochir Plast Chir. 2011 Oct;43(5):269-74. doi: 10.1055/s-0031-1286314. Epub 2011 Sep 20. Handchir Mikrochir Plast Chir. 2011. PMID: 21935843 Review. German.
Cited by
-
Oral doxycycline prevents skin-associated adverse effects induced by injectable collagenase in a rodent model of capsular contracture around silicone implants.PLoS One. 2022 Jul 6;17(7):e0270112. doi: 10.1371/journal.pone.0270112. eCollection 2022. PLoS One. 2022. PMID: 35793344 Free PMC article.
-
Collagenase Administration into Periodontal Ligament Reduces the Forces Required for Tooth Extraction in an Ex situ Porcine Jaw Model.J Funct Biomater. 2022 Jun 8;13(2):76. doi: 10.3390/jfb13020076. J Funct Biomater. 2022. PMID: 35735930 Free PMC article.
-
Treatment of dupuytren disease with injectable collagenase in a veteran population: a case series at the department of veterans affairs new jersey health care system.Eplasty. 2014 Mar 27;14:e13. eCollection 2014. Eplasty. 2014. PMID: 24741384 Free PMC article.
-
The Prognosis of Arthrofibroses: Prevalence, Clinical Shortcomings, and Future Prospects.Trends Pharmacol Sci. 2021 May;42(5):398-415. doi: 10.1016/j.tips.2021.02.007. Epub 2021 Mar 29. Trends Pharmacol Sci. 2021. PMID: 33795150 Free PMC article. Review.
-
Site-specific keloid fibroblasts alter the behaviour of normal skin and normal scar fibroblasts through paracrine signalling.PLoS One. 2013 Dec 9;8(12):e75600. doi: 10.1371/journal.pone.0075600. eCollection 2013. PLoS One. 2013. PMID: 24348987 Free PMC article.
References
-
- Thurston AJ. Dupuytren's disease. Journal of Bone and Joint Surgery - Series B. 2003;85:469–477. - PubMed
-
- Caufield RJ, Edwards SG. Dupuytren disease: An update on recent literature. Current Orthopaedic Practice. 2008;19:499–502.
-
- Dupuytren G. Permanent retraction of the fingers, produced by an affection of the palmar fascia. Lancet. 1834;2:222–225.
-
- Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol. 2010;6:715–726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

